Atypical and Anaplastic Meningiomas: Risk Factors, Classification and Management Choice
Wael K Zakaria
Department of Neurosurgery, Mansoura University, Mansoura, Egypt
Published Date: 2023-06-08DOI10.36648/2471-9633.7.1.12
Wael K Zakaria*, Samer Serag Eldin, Abd Elaziz Ismail and Mahmoud Saad
Department of Neurosurgery, Mansoura University, Mansoura, Egypt
- *Corresponding Author:
- Wael K Zakaria
Department of Neurosurgery, Mansoura University, Mansoura,
Egypt,
E-mail: drwaelmusa@yahoo.com
Received date: May 08, 2023, Manuscript No. IPJNCS-23-16570; Editor assigned date: May 10, 2023, PreQC No. IPJNCS-23-16570 (PQ); Reviewed date: May 18, 2023, QC No. IPJNCS-23-16570; Revised date: May 22, 2023, Manuscript No. IPJNCS-23-16570 (R); Published date: May 26, 2023, DOI: 10.36648/2471-9633.7.1.12
Citation: Zakaria WK, Eldin SS, Ismail AE, Saad M (2023) Atypical and Anaplastic Meningiomas: Risk Factors, Classification and Management Choice. Neurosurg Vol.7 No.1:12.
Abstract
Background: Non benign meningiomas include atypical type (WHO grade II tumor) and anaplastic type (WHO grade III tumor). Usually, Gross Total Resection (GTR) at the time of diagnosis considered as the line of management, but subsequent prognosis and optimal management remain unclear.
Methods: A retrospective study of 59 patients whom diagnosed as atypical or anaplastic meningioma in our institution from March 2006 to August 2022. Risk factors as age, sex and tumor location, extent of tumor resection were analyzed to determine their impact on tumor progression.
Results: This study included 59 newly diagnosed patients with malignant meningioma (40 females and 19 males, median age 51 years, range 3 to 80 years); attending to Mansoura University Hospital (MUH), 62.7% of them were in convexity, 100.0% of cases underwent total surgical resection and postoperative radiotherapy. Recurrence occurred in 27.1% of cases mainly in anaplastic with significant P value. Demographic data as age, gender, tumor location not show significant difference in atypical and malignant type and not considered as significant risk factors. The anaplastic meningioma was significant risk factor than the atypical type.
Conclusion: Patients with an anaplastic meningioma may develop a recurrent tumor than an atypical type. The anaplastic meningioma was significant risk factor for shorter overall survival and for shorter disease free survival. Radical surgical excision of the tumor or administration of adjuvant radiotherapy following initial incomplete surgical resection appears crucial for long-term treatment.
Keywords
Anaplastic; Atypical meningioma; GTR
Introduction
In 1922, Harvey Cushing described the term meningioma firstly in his publication as meningeal arising tumors in the spinal cord and brain and he found these tumors arise from the arachnoid cap cells [1-3]. Meningiomas considered as the most`existing primary intracranial extra axial tumors with an incidence of 2.3–8.3 in 100,000 [4-6]. Usually, meningiomas are benign in nature and slowly growing tumor (80%), but there are malignant types as atypical (15%-20%) and anaplastic (1%-3%) varieties. Recurrence rate of meningiomas usually low but if it happened, patients will have bad clinical prognosis and higher mortality [5,7-9].
There are many risk factors for malignant meningioma as sex, age, extent of tumor resection, location of tumor and prior radiation. The peak of meningioma’s incidence happened around the 6th and 7th decades of life but the malignant types exist in younger patients. Whereas benign meningioma’s are most common in females, the malignant meningioma occur almost in males with double incidence than female [10]. Meningioma characterized with progesterone and estrogen receptors in approximately 70%-80% of tumor, which explained the higher incidence in female more than male and support the theory of tumor growth due to hormonal component. Favorable prognosis associated with high levels of progesterone receptors, on the other hand, aggressive nature of tumor and high recurrence rate related to loss or absence of progesterone receptors [11-13]. Children characterized with very low incidence of meningioma’s, but it may happen in those exposed to cranial ionizing with an elevated risk of malignant types.
As regard the site, non-benign meningioma’s usually occurred in the cerebral convexities more than at the skull base. On the other hand when these meningioma’s types exist at the skull base, they characterized with lower recurrence rates and good prognosis than similar tumors exist in cerebral convexities [8,14].
Meningioma’s have WHO grading system based on many items as histopathology, tendency for recurrence and aggressiveness of the tumor and according the above items meningioma’s classifies into grade I (benign), grade II (atypical) and grade III (anaplastic) [7,8]. In the 2000, WHO classification of meningioma’s not considered invasion of the brain parenchyma as criterion for non-benign meningioma’s; but later researchers found that the tumor behavior and high risk of tumor recurrence associated with brain invasion. So in 2007, WHO revised their prior classification to include invasion of brain parenchyma as a separate criterion for malignant types of meningioma’s [7]. This revision lead to increase the incidence of grade II meningioma’s from 7 to 15%–20% [7,8,14]. In WHO 2016 grading, we not found any new changes in grading criteria. Pathological criteria for atypical and anaplastic meningioma’s collected in the below Table 1 [15].
| Atypical | Anaplastic |
|---|---|
| 4–19 mitotic figures per 10 high power microscope field | 20 or more mitotic figures per 10 high power microscope fields |
| Invasion of brain parenchyma, or exist of 3 of the following 5 histologic features: Hyper cellularity, small cells with high nuclear to cellular ratio, pattern less sheet-like growth, prominent nucleoli and spontaneous or geographic foci of necrosis | Frank an aplasia with focal, or diffuse loss of meningothelial differentiation and their cytology often resembles carcinoma, sarcoma, or melanoma |
| The chordoid and clear cell types of meningioma’s are also considered atypical | The papillary and rhabdoid subtypes are also classified as anaplastic |
Table 1: Histological features of high grade meningioma.
Surgical tumor excision is the primary treatment for high grade meningioma. Small sized, asymptomatic meningioma’s with benign features may be monitored or treated with stereotactic radiotherapy. In 1957, Donald Simpson described his classification related to extent of resection [16]. His classification has five categories upon the extent of resection Table 2.
| Simpson grade | |
| Grade 0 | Total tumor resection with 2–3 cm more from the site of tumor insertion |
| Grade I | Total tumor resection with dural attachment and abnormal bone |
| Grade II | Total tumor resection with dural attachment coagulation |
| Grade III | Total tumor resection without dural attachment coagulation or resection |
| Grade IV | Partial tumor resection |
| Grade V | Biopsy only |
Table 2: Simpson grading for extent of meningioma resection.
Generally, Gross Total Tumor Resection (GTR) considered as Simpson Grades I–III, while subtotal tumor resection considered as Simpson Grades IV–V [6,9,16-18]. Recently, a sixth grade category (Grade 0) was added when complete tumor resection with 2-3 cm more from the site of tumor insertion, with good outcome [19].
Radiotherapy considered as an effective line of management for meningioma’s. Literature based evidence concise that, adjuvant radiotherapy is usually an important and recommended line of management of incomplete resected grade II meningioma’s and for grade III meningioma’s regardless how much the extent of tumor resection [20-22]. However, in atypical meningioma’s patient’s with completely tumor resection, postoperative radiotherapy remains considerable debate and its depend upon physician preference [4,14,17,23-25].
Materials and Methods
Clinical material
In this study, we retrospectively reviewed the data of all patients who were diagnosed as malignant meningioma in Neurosurgery Department, Mansoura University Hospital (MUH) from March 2006 to August 2022. This study included 59 newly diagnosed patients with malignant meningioma (40 females and 19 males, with the median age 51 years, ranged from 3 to 80 years). We reviewed all pathological reports of the patients. Data were collected from our patients archiving system. All patients data used in this study as age, gender, site of tumor, postoperative radiotherapy and extent of surgical tumor resection listed in Table 3. We evaluate in this work the correlation between the above prognostic factors and recurrence rate, Overall Survival (OS) and Disease-Free Survival (DFS). As regard the extent of tumor resection, we include in this study all patients with total tumor resection which collected from operative data or postoperative Magnetic Resonance Imaging (MRI). Postoperative follow up patient’s data was collected from computerized data system in outpatient clinics.
| Patients | ||
| Median age | 51.0 (3-80) | |
| Gender | Male, (N (%)) | 19 (32.2%) |
| Female, (N (%)) | 40 (67.8%) | |
| Type | Atypical, (N (%)) | 47 (79.7%) |
| Anaplastic, (N (%)) | 12 (20.3%) | |
| Site | Convexity, (N (%)) | 37 (62.7%) |
| Parasagittal, (N (%)) | 11 (18.6%) | |
| Sphenoid, (N (%)) | 3 (5.1%) | |
| Falx, (N (%)) | 2 (3.4%) | |
| Tentorial, (N (%)) | 3 (5.1%) | |
| Olfactory, (N (%)) | 3 (5.1%) | |
| Surgical resection | N (%) | 59 (100.0%) |
| Radiotherapy | N (%) | 59 (100.0%) |
| Recurrence | N (%) | 16 (27.1%) |
Table 3: Patients’ characteristics among studied groups.
Methods
The age of patient at diagnosis defined as the date of first operation for malignant meningioma. The extent of surgical tumor resection was obtained by using the Simpson grading scale and depend on the operative note and post-operative radiology films. We defined tumor recurrence as a finding of tumor recurrence in post-operative radiology in case of total resection.
Statastical analysis
Results
This study included 59 newly diagnosed patients with meningioma (40 females and 19 males, the median age 51 years, ranged from 3 to 80 years); attending to Mansoura University Hospital (MUH), 62.7% of them were in convexity, 100.0% of cases underwent total surgical resection and postoperative radiotherapy. Recurrence occurred in 27.1% of cases. Prognostic factors as age, gender and tumor location and tumor types included in Table 3.
As regard meningioma type, anaplastic type had significantly higher recurrence rate compared to patients with atypical meningioma. Otherwise no other significant could be detected including demographic data as age, gender, tumor location (Table 4).
| Parameters | Atypical (n=47) |
Anaplastic (n=12) |
P-value | ||
|---|---|---|---|---|---|
| Age* | Median (Min-Max) | 52.0 (13-80) | 46.0 (3-63) | 0.095 | |
| Age group | <20 | Count (%) | 1 (2.1%) | 1 (8.3%) | 0.219 |
| 20-40 | Count (%) | 4 (8.5%) | 3 (25.0%) | ||
| 41-60 | Count (%) | 31 (66.0%) | 7 (58.3%) | ||
| 61-80 | Count (%) | 11 (23.4%) | 1 (8.3%) | ||
| Gender | Male | Count (%) | 13 (27.7%) | 6 (50.0%) | 0.139 |
| Female | Count (%) | 34 (72.3%) | 6 (50.0%) | ||
| Site | Convexity | Count (%) | 28 (59.6%) | 9 (75.0%) | 0.739 |
| Parasagittal | Count (%) | 9 (19.1%) | 2 (16.7%) | ||
| Sphenoid | Count (%) | 2 (4.3%) | 1 (8.3%) | ||
| Flax | Count (%) | 2 (4.3%) | 0 (0.0%) | ||
| Tentorial | Count (%) | 3 (6.4%) | 0 (0.0%) | ||
| Olfactory | Count (%) | 3 (6.4%) | 0 (0.0%) | ||
| Surgical resection | Count (%) | 47 (100.0%) | 12 (100.0%) | ||
| Radiotherapy | Count (%) | 47 (100.0%) | 12 (100.0%) | ||
| Recurrence | Count (%) | 9 (19.1%) | 7 (58.3%) | 0.006 | |
| Note: Mann-Whitney test*; chi square test (fisher’s exact test), P between both groups. Significant (P value<0.05). |
|||||
Table 4: Comparison of clinic pathological characteristics as regard meningioma type.
COX Regression analysis conducted for prediction of shorter overall survival, using age, gender, tumor location and type as covariates. The anaplastic meningioma was significant risk factor for shorter OS (Table 5).
| Univariate analysis | |||||
|---|---|---|---|---|---|
| p | HR | 95% CI | |||
| Age | 0.8 | 1.004 | 0.958 | 1.051 | |
| Gender | 0.523 | 0.662 | 0.187 | 2.348 | |
| Tumor location | Others | ref | 1 | - | - |
| Convexity | 0.972 | 0.977 | 0.196 | 4.818 | |
| Parasagittal | 0.841 | 1.223 | 0.172 | 8.704 | |
| Anaplastic vs. atypical | 0.005 | 6.23 | 1.753 | 22.143 | |
| Note: HR: Hazard Ratio; CI: Confidence Interval; COX regression was used. | |||||
Table 5: Cox regression analysis for prediction of shorter OS.
COX Regression analysis conducted for prediction of shorter disease free survival, using age, gender, and type as covariates.
The anaplastic meningioma was significant risk factor for shorter DFS (Table 6).
| Univariate analysis | |||||
|---|---|---|---|---|---|
| p | HR | 95% CI | |||
| Age | 0.8 | 1.004 | 0.958 | 1.051 | |
| Gender | 0.523 | 0.662 | 0.187 | 2.348 | |
| Tumor location | Others | ref | 1 | - | - |
| Convexity | 0.972 | 0.977 | 0.196 | 4.818 | |
| Parasagittal | 0.841 | 1.223 | 0.172 | 8.704 | |
| Anaplastic vs. atypical | 0.005 | 6.23 | 1.753 | 22.143 | |
| Note: HR: Hazard Ratio; CI: Confidence Interval; COX regression was used. | |||||
Table 6: Cox regression analysis for prediction of shorter DFS.
Survival analysis
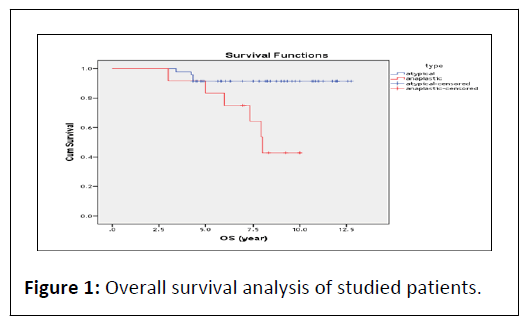
At the end of follow up period (12 year), OS of studied patients estimates 87.6% at 6 year interval and 80.2% at 12 year interval. As regard meningioma type, OS estimates 91.5% at 6 year interval and 91.5% at 12 year interval in atypical group, also OS estimates 75.0% at 6 year interval and 42.9% at 12 year interval in anaplastic group with significant difference between 2 groups (P<0.001) (Figure 1).
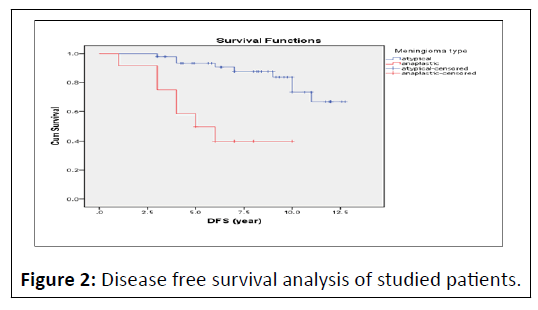
At the end of follow up period (12 year), DFS of studied cases estimates 79.9% at 6 year interval and 59.5% at 12 year interval. As regard meningioma type, DFS estimates 90.7% at 6 year interval and 66.6% at 12 year interval in atypical group, also DFS estimates 40.0% at 6 year interval and 40.0% at 12 year interval in anaplastic group with significant difference between 2 groups(P<0.001) (Figure 2).
Discussion
Malignant meningioma is rare tumor and its optimal treatment is still debit in guideline. In this study, we collect data for long period to evaluate the clinical outcome and expected prognostic factors that affect the overall survival and disease free survival rates of atypical and anaplastic meningioma.
Benign grade I meningioma’s are considered as 90% of all meningioma tumors. Atypical meningioma’s represent 4.7%– 10% of all meningioma’s, while the anaplastic type account for only 1%–2.8% of all meningioma’s [1-3]. In the diagnosis of malignant meningioma’s we based on the WHO (2016) classification and its upgrading included brain invasion to the previous histological characteristic which mentioned in Table 1 [6].
Many literature studies suggests that the extent of tumor resection, based on Simpson grade system, considered as the most important prognostic factor for good outcome in a malignant meningioma patients [6,26]. In our series, we select all cases with total tumor resection (Simpson grade I resection) to know the effect of the different prognostic factors associated with OS, DFS as the age of patient at the time of tumor diagnosis, site of tumor, and post-operative radiotherapy.
Age
There was no statistical significance of age of the patients as prognostic factors. However we find the age group 41-60 years old is the most age group for malignant meningioma in our study with high recurrence rate 56.25% which is different to results of Champeaux, et al., [25] reported that patients younger than 57 years had fewer operations than those above 57 years old since recurrence and OS were shown to be associated with age at tumor diagnosis [25]. In different study, Aghi, et al., report that age at diagnosis is closely associated with the overall survival of atypical meningioma [17].
Gender
As regard the gander, we not find any statistical significant between the atypical and the anaplastic type and this different from some literature studies whom find that female gender have been related to poor prognosis and characterized radiological features such as peritumoral edema, heterogeneous enhancement in post contrast studies and intra tumoural cyst formation have been implicated with lower median recurrencefree survival [4,5].
Tumor location
We found that the majority of malignant meningioma’s occurs in convexity of cerebrum rather than skull base with the same results of Hug, et al., [27]. On the other hand, the comparison between recurrence rate in convexity and skull base malignant meningioma, Hug, et al., [27] found that the tumor recurrence rate was increased in skull base malignant meningioma and this not meet our results and this may due to the limited number of malignant skull base meningioma’s include in our study (5 cases).
Adjuvant radiotherapy
In our study, all patients sent for receiving post-operative radiotherapy due its significant as important line of treatment and this was compatible with literature studies which found that post-operative radiation is a very important line of the treatment of non-benign meningioma’s. Five year progressionfree survival increased from 15%-80% when external beam radiotherapy was added to tumor resection for mom benign meningioma [14,24]. Stereotactic radiosurgery is no longer used for malignant meningioma’s due to the possibility of margin inclusion in irradiation field with external beam radiotherapy. However, Lubgan, et al., found an excellent outcome with gamma knife radiotherapy when tailored as an adjuvant therapy after complete tumoral removal or as definitive treatment regime [28,29].
Conclusion
Atypical (grade II) and anaplastic (grade III) meningioma’s remain challenging diseases, and optimal guidelines treatment is current unavailable. Anaplastic type had significantly higher recurrence rate compared to patients with atypical meningioma. Otherwise no other significant could be detected including demographic data as age, gender, tumor location. The upcoming studied must focus on the biological signature of these malignant tumor, as our reported prognostic factors still statistically insignificant.
References
- Barthélemy EJ, Sarkiss CA, Lee J, Shrivastava RK (2016) The historical origin of the term “meningioma” and the rise of nationalistic neurosurgery. J Neurosurg 125: 1283-1290.
[Crossref], [Google scholar], [Indexed]
- Cushing H (1922) The meningiomas (dural endotheliomas): Their source and favored seats of origin (Cavendish Lecture). Brain 45: 282-316.
- Pendleton C, Olivi A, Brem H, Quiñones-Hinojosa A (2013) Harvey Cushing’s early treatment of meningiomas: The untold story. World Neurosurg 80: 217-221.
[Crossref], [Google scholar], [Indexed]
- Hammouche S, Clark S, Wong AHL, Eldridge P, Farah JO (2014) Long-term survival analysis of atypical meningiomas: Survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir 156: 1475-1481.
[Crossref], [Google scholar], [Indexed]
- Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, et al. (2016) CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol 18: v1-v75.
[Crossref], [Google scholar], [Indexed]
- Riemenschneider MJ, Perry A, Reifenberger G (2006) Histological classification and molecular genetics of meningiomas. Lancet Neurol 5: 1045-1054.
[Crossref], [Google scholar], [Indexed]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, et al. (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114: 97-109.
[Crossref], [Google scholar], [Indexed]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, et al. (2016) The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol 131: 803-820.
[Crossref], [Google scholar], [Indexed]
- Rogers L, Gilbert M, Vogelbaum MA (2010) Intracranial meningiomas of atypical (WHO grade II) histology. J Neurooncol 99: 393-405.
[Crossref], [Google scholar], [Indexed]
- James MF, Han S, Polizzano C, Plotkin SR, Manning BD, et al. (2009) NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol 29: 4250-4261.
[Crossref], [Google scholar], [Indexed]
- Kane AJ, Sughrue ME, Rutkowski MJ, Shangari G, Fang S, et al. (2011) Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer 117: 1272-1278.
[Crossref], [Google scholar], [Indexed]
- Pravdenkova S, Al-Mefty O, Sawyer J, Husain M (2006) Progesterone and estrogen receptors: Opposing prognostic indicators in meningiomas. J Neurosurg 105: 163–173.
[Crossref], [Google scholar], [Indexed]
- Wiemels J, Wrensch M, Claus EB (2010) Epidemiology and etiology of meningioma. J Neurooncol 99: 307-314.
[Crossref], [Google scholar], [Indexed]
- Liang R-F, Xiu YJ, Wang X, Li M, Yang Y, et al. (2014) The potential risk factors for atypical and anaplastic meningiomas: Clinical series of 1,239 cases. Int J Clin Exp Med 7: 5696-5700 [Crossref],
[Google scholar], [Indexed]
- Prayson R (2012) Neuropathology. (2nd ed), Elsevier Saunders Philadelphia, PA: 513–60.
- Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20: 22-39.
[Crossref], [Google scholar], [Indexed]
- Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjani S, et al. (2009) Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 64: 56-60.
[Crossref], [Google scholar], [Indexed]
- Condra KS, Buatti JM, Mendenhall WM, Friedman WA, Marcus RB, et al. (1997) Benig meningiomas: Primary treatment selection affects survival. Int J Radiat Oncol Biol Phys 39: 427-436.
[Crossref], [Google scholar], [Indexed]
- Mooney MA, Abolfotoh M, BiWL, Tavanaiepour D, Almefty RO, et al. (2020) Is falcine meningioma a diffuse disease of the falx? Case series and analysis of a “Grade Zero” Resection. Neurosurgery 87: 900-909.
[Crossref], [Google scholar], [Indexed]
- Cho M, Joo JD, Kim IA, Han JH, Oh CW, et al. (2017)The role of adjuvant treatment in patients with high-grade meningioma. J Korean Neurosurg Soc 60:527–533.
[Crossref], [Google scholar], [Indexed]
- Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, et al. (2016) EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 17: 383–391.
[Crossref], [Google scholar], [Indexed]
- Wang YC, Chuang CC, Wei KC, Chang CN, Lee ST, et al. (2016) Long term surgical outcome and prognostic factors of atypical and malignant meningiomas. Sci Rep 6: 35743.
[Crossref], [Google scholar], [Indexed]
- Mair R, Morris K, Scott I, Carroll TA (2011) Radiotherapy for atypical meningiomas. J Neurosurg 115: 811-819.
[Crossref], [Google scholar], [Indexed]
- Graffeo CS, Leeper HE, Perry A, Uhm JH, Lachance DJ, et al. (2017) Revisiting adjuvant radiotherapy after gross total resection of World Health Organization grade II meningioma. World Neurosurg 103: 655-663.
[Crossref], [Google scholar], [Indexed]
- Champeaux C, Houston D, Dunn L (2017) Atypical meningioma. A study on recurrence and disease-specific survival. Neurochirurgie 63: 273-281.
[Crossref], [Google scholar], [Indexed]
- Magill ST, Young JS, Chae R, Aghi MK, Theodosopoulos PV (20108) McDermott MW. Relationship between tumor location, size and WHO grade in meningioma. Neurosurg Focus 44: E4.
[Crossref], [Google scholar], [Indexed]
- Hug EB, Devries A, Thornton AF, Munzenride JE, Pardo FS, et al. (2000) Management of atypical and malignant meningiomas: Role of high-dose, 3D-conformal radiation therapy. J Neurooncol 48: 151-160.
[Crossref], [Google scholar], [Indexed]
- Lubgan D, Rutzner S, Lambrecht U (2017) Stereotactic radiotherapy as primary definitive or postoperative treatment of intracranial meningioma of WHO grade II and III leads to better disease control than stereotactic radiotherapy of recurrent meningioma. J Neurooncol 134: 407-416.
[Crossref], [Google scholar], [Indexed]
- Komotar RJ, Iorgulescu JB, Raper DMS, Holland EC, Beal K, et al. (2012) The role of radiotherapy following gross-total resection of atypical meningiomas. J Neurosurg 117: 679-686.
[Crossref], [Google scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences