Capsule Granulation Tissue Harvested From Abdominal Region Used as Dural Autologous Graft
Marco Aurelio Rendón Medina
Hospital General de Mexico, Mexico
- *Corresponding Author:
- Marco Aurelio Rendón Medina
Hospital General de Mexico, Surgery, Balmis 148
Balmis 148, 03300, Mexico, Ciudad de México
Ciudad de México 06726, Mexico.
Tel: +3338152872, +5551453785
Fax: +5551453785
E-mail: dr.rendon1989@gmail.com
Received date: June 27, 2017; Accepted date: July 19, 2017; Published date: July 24, 2017
Citation: Medina MAR (2017) Capsule Granulation Tissue Harvested From Abdominal Region Used as Dural Autologous Graft. Neurosurg. Vol. 1 No. 3:21.
Abstract
In neurosurgery neural defects are a common problem, many etiologies compromise dura mater integrity. These scenarios had demonstrated the need for a dural substitute. Multiple substitutes had been proposed: autografts, allografts, xenografts and synthetic materials. None of them fulfill all the "ideal substitute" enquiries. The hypothesis of this paper is supported by previous work of Tomohisa et al. And Campbell et al. Tomohisa et al presented 12 cases were synthetic substitutes had infected tissue and the need of removal was inevitable. Fortunately, the capsule was protecting brain parenchyma, and the absence of CSFL give them the option of closing with no further duroplasty. They suggest that in these scenarios where infection prevents the option any graft, the preexisting capsule works as an appropriate substitute. In the other paper, a new vascular graft was presented, where sylastic tube were placed in rabbits and mice, then harvested after two weeks and used the capsule of granulation tissue as an autologous vascular graft. This autologous substitute could work as an adequate scaffold for graft integration in dura mater.
https://marsbahislinki.com https://marsbetgiris.com https://mobilebahiss.com https://1xbetsgirisi.com https://onwinegiris.com https://betistegiris.com https://piacasinogiris.com https://holiganbahiss.com https://holiganbetting.com https://girisholigan.com https://mostbetegiris.com https://mariobetoyna.com https://meritgirisi.com https://meritkinge.com https://kingmerite.com https://nanogiris.com https://casinoplusa.com https://casinoplusgiris.com https://betriyal.net https://pluscasinogiris.com
Keywords
Dural substitute; Autologous graft; Neurosurgery; Biological duramater substitute
Introduction
In neurosurgery neural defects are a common problem, many etiologies compromise dura mater integrity. These scenarios had demonstrated the need for a dural substitute. Etiologies affecting dura mater are tumor invasion, congenital meninges defects, traumatism other pathologies [1]. Many experimental studies had been realized. Still, no ideal dural substitute is available [2]. Dural decent substitute is necessary to avoid complications. For example, Cerebrospinal Fluid Leakage (CSFL) resulting in fistula, cerebral herniation, pneumoencephalus, pseudomeningocele, adhesions among others can be present [3]. Multiple substitutes had been proposed: Autografts, allografts, xenografts and synthetic materials [4]. None of them fulfill all the “ideal substitute” enquiries, which are: inexpensive, available, strong, malleable, easily managed, inert, nontoxic, watertight barrier, don’t create adhesions to brain parenchyma or cranium, safe of infectious diseases [5]. Most of the research is in xenografts and synthetic materials. Many advantages had been extensively described in the literature. In the other hand, these substitutes have the specific disadvantage of creating complications such as infection or allergic reactions [6,7].
For fortune these complications are uncommon, but once they appear surgeon options are narrowed nearly to zero. The hypothesis of this paper is supported by previous work of Nagasao et al. [6] and Campbell et al. [8]. Nagasao et al. [6] presented 12 cases were synthetic substitutes had infected tissue, and the need of removal was inevitable. Fortunately, the capsule was protecting brain parenchyma and the absence of CSFL gives them the option of closing with no further duroplasty. They suggest that in these scenarios where infection prevents the option any graft, the preexisting capsule works as an appropriate substitute [6]. In the other paper, Campbell et al. [8] present a new vascular graft, they put sylastic tube in rabbits and mice, then harvested after two weeks and used the capsule of granulation tissue as an autologous vascular graft [8]. This autologous substitute could work as an adequate scaffold for graft integration in dura mater. The search for the ideal substitute after nearly 100 years still is on, and as we had described every graft has its pros and cons. We want to add a tool, into the tool box for development countries or well when a synthetic substitute presents infection. In this paper, the objective is to describe a new alternative. We will introduce the hypothesis of autologous fibrous tissue harvested from the subcutaneous tissue, as a dural graft. This is just a theoretical paper; we want to encourage other researchers to conduct this experiment.
Materials and Methods
Sylastic peritoneal or subcutaneous implant
We suggest using 1 × 1 × 0.3 cm squared sylastic implant or well half spheres with a radius of 1 cm (Figure 1). The implant has to be sterilized and packaged. Campbell et al. [8] used 4 sylastic tubes to have spares in the second surgery.
In vitro
Observation of microstructure: We suggest to use observation by scanning electron microscopy (SEM to Measure the pore diameter and thickness.
Measurements of mechanical strength
The mechanical strength of the fibrous capsule should be performed under 37°C. Measuring tensile strength and elongation breakage. Multiple methods to do it had been described in the literature [1-4,9]. Water retention also is strongly advised to document [3,9]. It can be measured with the formula:

Wwet stands for weight documented after leaving the samples in saline solution for 24 h. Then leave the samples to dry for 24 h and then weigh them and register it as Wdry [3].
In vivo stage 1: Implantation surgical technique
This experiment can be conducted in New Zealand Rabbits, Wistar rats or canine model. We suggest conducting the investigation in New Zaeland Rabbit model. Be sure to respect all the local authorities’ regulations. In Mexico are NOM ZOO099 and International Council for Laboratory Animals ICLAS. In this experiment design, would need a two staged surgery. The first when the sylastic material are implanted in the subcutaneous tissue or in the peritoneal cavity. So either dermis dissection or well laparotomy should be performed.
In vivo stage 2: Harvesting the implants and conduction craniectomy
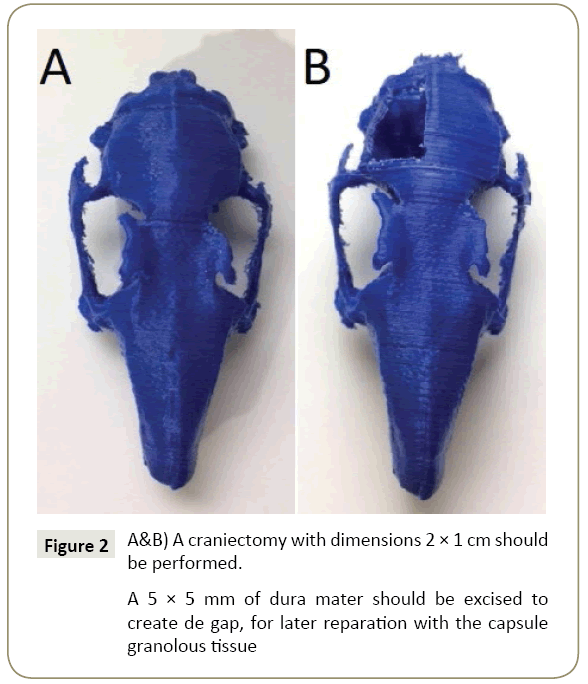
After two weeks, the implants should be ready for harvesting [9]. Wang et al. [9] collected the implants and everted the borders for implantation. We suggest that the inner part of the capsule (the one that has in contact with the sylastic) is on the brain side of the implant. Standardized Craniectomy should be addressed measuring 2 × 1 cm in the right parietal region (Figure 2). Opening the dura under microscopic observation then remove 5 × 5 mm of dura mater. Later harvest 6x6 mm fibrous capsule and suturing it with Nylon 7-0 monofilament USP in interrupted knots [3,4]. The cranial bone defect can be left open or can be closed with the bone autograft, but it has to be standardized in every craniectomy [2]. At this stage, a local antibiotic such as Penicillin powder can be used [1]. The skin can be sutured with Nylon monofilament 4-0 USP.
Postoperative observation
Postsurgical outcomes must be documented, such as eating or drinking abnormally, animal activity, infection or CFSL.
Staged standardized leakage documentation
We recommend conducting fluid leakage pressure as Fillipi et al. [10] described. Standardized dates have to be defined for example days 10, 21 and 30 days and subsequently performed.
General anesthesia should be given, then reopen the cranial wound (optional: Removing skull), making an incision in the native dura (respecting the autologous graft). Then the insertion of a catheter of 22 G into the cisterna magna fixing with an adhesive sealant, attaching the catheter to a pressure monitor and a continuous infusion device. Then a Fluorescein solution (0.9% saline with 0.05% sodium fluorescein) is perfused to the cisterna magna. With the assistance of ultraviolet illumination, any fluorescein leakage should be evident. The infusion should continue until the pressure reaches 100 mm Hg [5]. Alternatively Sandoval-Sánchez et al. method can be conducted [3].
Histological evaluation
All animals had to be anesthetized and then killed painlessly. We suggest the intravenous overdose of sodium pentobarbital at different time after the implantation, to document staged implant integration. The principal outcomes to register are adhesions, vascularity and integration [1,5]. Macroscopic adhesions assessment: can be graded by Park and Tator [11]. Systems: Grade 1: absence of adhesions, Grade 2: thin adhesions not converting the whole duraplasty site, Grade 3: contiguous adhesions covering the entire duraplasty site [2,5]. The vascularity of the duraplasty site as Maher et al. [5], with the number of vessels in a 100 μm2 cross sectional area. 0 represents zero to one vessel/100 μm2; 1 is two to four vessels/100 μm2 and 2 is five or more vessel/100 μm. Finally, the integration of the capsule should be documented as Matsumoto et al. [2]. It can be represented as follows 0 no integration, 1 integration of fibroblast, 2 only parts of the initial graft are detectable and 4 complete integration or neodura [2].
Results reported by Campbell et al. [8]
Campbell et al. [8] reported complete capsule of granulation tissue after 3 weeks of peritoneal implantation. Histological findings were a layer of connective tissue covered by several layers of cell-rich granulation tissue. A single layer of cells stained positively for von Willebrand factor, and electron microscopy confirmed their identification as mesothelial cells and they found high levels of collagen Types I and IV. Unfortunately, Campbell didn’t report wall thickness or pore diameter in SEM. Nagasao et al. [6] indicated that capsule founded was watertight and no CSFL was founded, but still would be valuable to conduct standardized staged leakage tests to know certainly for how long CSFL could be expected with this dural substitute.
Discussion
Extensive research has been addressed in search of an ideal dural substitute. Synthetic substitutes and xenografts are the vast majority of papers in the literature. When complications appear no ideal substitute is recommended to use. This autologous low cost graft could be an attractive prospect. The results reported by Campbell et al. [8] suggests that this fibrous capsule could be a suitable dura mater substitute. It could work as a scaffold for fibroblast and neovascularization, resulting in complete graft integration. The two staged surgical approach, could refute an initial approach in clinical applications. But synthetic substitutes not always are available due to high cost. Alternatively, other autologous grafts can be more suitable as galea-pericranium or fascia lata. Sabatino et al. [12] reported good results with galeapéricraium grafts in 92 patients with mean dural gaps big as 12 cm2 [12]. If this hypothesis proved correct, new technologies could be implemented to apply the advances in bioengineering and avoid the initial surgery. With new bioengineering techniques, collagen scaffolds can be 3D printed, complex tissues as skin are already possible [13]. This prospect could redefine the direction of dura mater substitute searching race. We want to encourage other researchers to conduct this project.
Conclusion
We presented how to conduct animal model, with an attractive prospect for autologous graft. We cannot present conclusion because we have not perfomed the experiment jet.
References
- Shi Z, Xu T, Yuan Y (2016) A New absorbable synthetic substitute with biomimetic design for dural tissue repair. Artif Organs. 40: 403-413.
- Matsumoto K, Nakamura T, Fukuda S, Sekine T, Ueda H, et al. (2001) A gelatin coated collagen-polyglycolic acid composite membrane as a dural substitute. ASAIO J 47: 641-645.
- Sandoval-sánchez JH, Ramos-zúñiga R, Anda SL De, López-dellamary F (2012) A new bilayer chitosan scaffolding as a dural substitute : Experimental evaluation. World Neurosurg 77: 577-582.
- Yamada K, Miyamoto S, Nagata I (1997) Development of a dural substitute from synthetic bioabsorbable polymers. J Neurosurg 86: 1012-1017.
- Maher CO, Anderson RE, McClelland RL, Link MJ (2003) Evaluation of a novel propylene oxide-treated collagen material as a dural substitute. J Neurosurg 99: 1070-1076.
- Nagasao T, Shinoda J, Horiguchi T, Kishi K (2011) Capsule formation can make secondary reconstruction of the dura mater unnecessary after cranial infection. J Craniofac Surg 22: 84-88.
- Foy AB, Giannini C, Raffel C (2008) Allergic reaction to a bovine dural substitute following spinal cord untethering. Case report. J Neurosurg Pediatr. 1: 167-169.
- Campbell JH, Efendy JL, Campbell GR (1999) Novel vascular graft grown within recipient’s own peritoneal cavity. Circ Res. 85: 1173-1178.
- Wang YF, Guo HF, Ying DJ (2013) Multilayer scaffold of electrospun PLA-PCL-collagen nanofibers as a dural substitute. J Biomed Mater Res - Part B Appl Biomater 101: 1359-1366.
- Filippi R, Derdilopoulos A, Axel Heimann DVM, Krummenauer F, Oliver Kempski AP, et al. Tightness of Duraplasty in Rabbits: A comparative study. Neurosurgery 46: 1470-1477
- Park YK, Tator CH (1998) Prevention of arachnoiditis and postoperative tethering of the spinal cord with gore-tex surgical membrane: An experimental study with Rats. Neurosurgery 42: 813-823.
- Sabatino G, Maria G, Pepa D (2014) Autologous dural substitutes : A prospective study. Clin Neurol Neurosurg 116: 20-23.
- Lee V, Singh G, Trasatti JP (2014) Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods 20: 473-484.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences