Clinical Outcome of Anterior Cervical Discectomy and Fusion versus Total Disc Replacement A Meta-Analysis of 2532 Cases
Stefan Alexander König and Uwe Spetzger
DOI10.21767/2471-9633.100014
Stefan Alexander König* and Uwe Spetzger
Klinikum Karlsruhe, Karlsruhe, Germany
- *Corresponding Author:
- Stefan Alexander König
M.D., Klinikum Karlsruhe
Karlsruhe, Germany
Tel: 0721 974-3501
E-mail: Koenig_de@web.de
Received: May 08, 2016; Accepted: May 23, 2016; Published: May 30, 2016
Citation: König SA, Spetzger U. Clinical Outcome of Anterior Cervical Discectomy and Fusion versus Total Disc Replacement – A Meta-Analysis of 2532 Cases. Neurosurg. 2016, 1:2.
Abstract
Purpose: Cervical total disc replacement (TDR) has become an alternative to fusion (ACDF) in the surgical treatment of cervical radiculopathy with the promise of preserving motion in the affected segment and of avoiding accelerated degeneration of the adjacent levels. Nowadays there are numerous prospective, randomised and controlled studies comparing the clinical outcome of patients with both therapeutical options. Since cervical prostheses are much more expensive than cages it is crucial to compare the clinical benefit of both implants on the basis of a larger amount of patient data.
Methods: A systematic data base search and study review identified 11 prospective, randomized and controlled studies that compared the clinical outcome after ACDF or TDR using at least the scores of NDI, VAS for neck pain and VAS for arm pain after a follow-up of at least 2 years. 2532 cases were included in the statistical analysis that compared the mean values of the clinical scores.
Results: There were no statistically significant difference between the ACDF group and the TDR group in the clinical outcome scores (NDI, VAS neck, VAS arm) 2 years after surgery although some of the included studies found differences.
Conclusion: ACDF and cervical TDR generate the same clinical results 2 years after surgery in regard to pain relief. Some of the included studies give proof of a better range of motion and less adjacent level degeneration in the TDR group. Thus, this more expensive option is only indicated in younger patients with soft-disc herniation.
Keywords
Cervical; Fusion; Total disc replacement; Clinical outcome; Meta-analysis
Introduction
For the surgical treatment of cervical spondylotic radiculopathy due to disc herniation anterior cervical discectomy and fusion (ACDF) has been the standard therapy for many years. Since the development of cervical prostheses in the 1990s total disc replacement (TDR) has become an alternative to preserve motion in the affected level especially in younger patients.
In the early days of TDR there were only studies with short followup periods and small patient populations. Thus, a sufficient medical and economical assessment of cervical prostheses has been difficult. From a medical point of view it is important to know if patients clinically benefit and if there is secondary bony fusion or not because these TDR implants are much more expensive compared to a cervical fusion cage [1-6]. Nowadays there are numerous prospective, randomised and controlled studies comparing the clinical outcome of patients with TDR or ACDF [7-17].
The purpose of this meta-analysis was to compare the clinical outcomes between ACDF and TDR for the surgical treatment of cervical spondylotic radiculopathy after a follow-up time of at least two years and to compare it with other analysises [1-3]. In our opinion a follow-up time of at least two years is necessary to identify cases with a slow secondary fusion after cervical TDR.
Methods
Inclusion criteria
Studies were included if they:
(1) Had a prospective, randomized and controlled study design;
(2) Had a minimum follow-up time of 2 years;
(3) Included patients with cervical spondylotic radiculopathy without myelopathy;
(4) Included patients >18 years of age.
Exclusion criteria
Studies were excluded if they:
(1) Did not have a prospective, randomized and controlled study design,
(2) Had a follow-up time of less than 2 years,
(3) Included patients with cervical spondylotic myelopathy,
(4) Did not use VAS and/or NDI for the measurement of clinical outcome.
Search for and selection of studies
The authors searched the databases of MEDLINE and the Cochrane Central Register of Controlled Trials. There was no restriction regarding the publication date or the language of the publication.
The databases were searched for the terms:
(1) Cervical spine OR radiculopathy OR degenerative disc disease OR nerve root compression;
(2) Total disc replacement OR prosthesis OR dynamic implant;
(3) Fusion OR anterior decompression and fusion OR ACDF;
(4) Clinical outcome OR JOA score OR neck disability index OR NDI OR visual analogue scale OR VAS.
The main author conducted the search of the databases using the search terms mentioned above. Afterwards the titles and abstracts of all search results were reviewed to exclude unrelated studies. In the next step all authors reviewed the selected publications according to the inclusion and exclusion criteria. The reviewers were not blinded to the authors or the journal of the studies. After the first review process all authors reviewed the full text of all studies that were finally included into the meta-analysis.
Data acquisition
The following data were collected from each study and stored in a tabular form:
(1) Study ID;
(2) Study design;
(3) Number of patients;
(4) Patient demographics;
(5) Follow-up time;
(6) Type of TDR;
(7) Type of fusion;
(8) Japanese Orthopedic Association (JOA) score;
(9) Neck Disability Index (NDI);
(10) Visual Analogue Scale (VAS) score for neck pain;
(11) VAS score for arm pain.
Statistical analysis
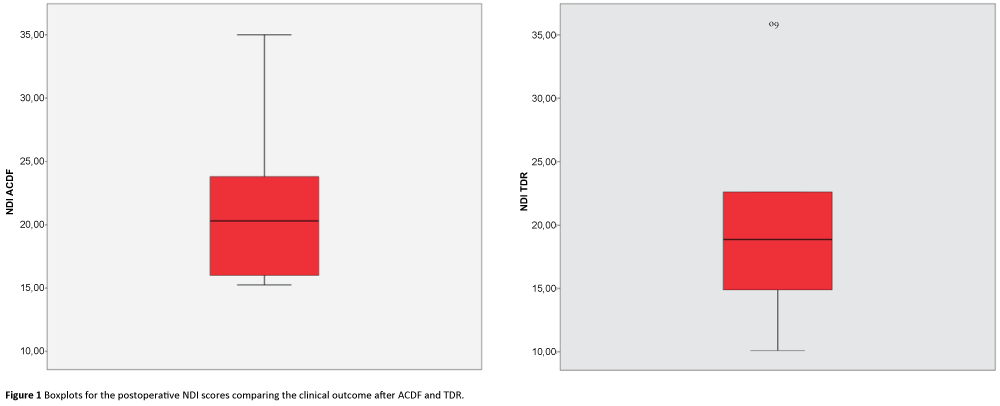
The statistical data analysis was performed using SPSS Statistics, version 23.0.0.2 (IBM Corporation, Armonk, NY, USA). Mean, standard deviation, standard error of the mean, confidence interval, mean differences, standard error of the difference, T-value and two-tailed p-values were calculated. Boxplots were generated to visualize the data and mean values from the ACDF and TDR groups (Figures 1-3).
Results
Search results
The search in both databases identified 321 studies. Of these studies 279 had to be excluded because they were biomechanical investigations, reviews, meta-analysises, non-human experimental studies or case reports.
The remaining 42 studies underwent full text review with special emphasis on the inclusion and exclusion criteria mentioned above. Finally, 11 studies could be identified that met all of the defined criteria (Table 1) [7-17].
| Study ID, year |
Study design | Number of patients | Patient age statistics (years) |
Follow-up time (months) |
Type of TDR, number of patients |
Type of fusion, number of patients |
JOA score | NDI at endpoint |
VAS neck at endpoint |
VAS arm at endpoint |
|---|---|---|---|---|---|---|---|---|---|---|
| Sasso et al. [1] | Prospective Randomized Controlled Multicenter |
115 | TDR: 42.5 (± 7.8) ACDF: 46.1 (±7.8) |
24 | BRYAN®(n=56) | Fibular allograft and anterior plate(n=59) | - | TDR: 36 ACDF: 35 |
TDR: 36 ACDF: 35 |
TDR: 36 ACDF: 35 |
| Murrey et al. [2] | Prospective Randomized Controlled Multicenter |
209 | TDR: 42.1 (± 8.4) ACDF: 43.5 (± 7.1) |
24 | ProDisc-C®(n=103) | Allograft bone and plate (n=106) | - | TDR: 21.4 ACDF: 20.5 |
TDR: 36 ACDF: 34 |
TDR: 20 ACDF: 18 |
| Garrido et al. [3] | Prospective Randomized Controlled |
47 | TDR: 40 ACDF: 43 |
48 | Bryan® (n=21) | Heterologous bone graft (n=26) | - | TDR: 10.1 ACDF: 15.9 |
TDR: 13.6 ACDF: 28.1 |
TDR: 10.8 ACDF: 21.7 |
| Coric et al.[4] | Prospective Randomized Controlled Multicenter |
269 | TDR: 43.7 (27-61) ACDF: 43.9 (23-62) |
24 | Kineflex/C®(n=136) | Allograft and plate(n=133) | - | TDR: 22.6 ACDF: 23.4 |
TDR: 23.6 ACDF: 24.2 (cumulative neck + arm) |
|
| Zhang et al.[5] | Prospective Randomized Controlled Multicenter |
120 | TDR: 44.8 (32-59) ACDF: 45.6 (31-62) |
24 | Bryan® (n=60) | Fibular allograft and anterior plating (n=60) | - | TDR: 14.89 ± 2.90 ACDF: 15.25 ± 3.77 |
TDR: 19.07 ± 5.02 ACDF: 21.45 ± 4.85 |
TDR: 16.20 ± 3.76 ACDF: 17.34 ± 4.76 |
| Phillips et al.[6] | Prospective Randomized Controlled |
342 | TDR: 45.3 (18-68) ACDF: 43.7 (20-63) |
24 | PCM Cervical Disc® (n=218) | Allograft and plate (n=185) | - | TDR: 25 ACDF: 30 |
TDR: 28 ACDF: 30 |
|
| Vaccaro et al.[7] | Prospective Randomized Controlled Multicenter |
380 | TDR: 47 (30-63) ACDF: 44 (32-62) |
24 | SECURE-C® (n=240) | Allograft and plate (n=140) | - | TDR: 12 ACDF: 16 |
TDR: 14 ACDF: 20 |
TDR: 7 ACDF: 9 |
| Burkus et al. [8] | Prospective Randomized Controlled Multicenter |
541 | TDR: 43.3 (25-72) ACDF: 43.9 (22-73) |
84 | Prestige® (n=276) | N/A (n=265) | - | TDR: 18.1 ACDF: 23.8 |
TDR: 13.1 ACDF: 19.4 |
TDR: 12.7 ACDF: 15.0 |
| Hisey et al.[9] | Prospective Randomized Controlled Multicenter |
245 | TDR: 43.3 (± 9.2) ACDF: 44.0 (± 8.2) |
24 | Mobi-C®(n=164) | Allograft bone and plate (n=81) | - | TDR: 16 ACDF: 18 |
TDR: 17.34 ACDF: 19.36 |
TDR: 13.6 ACDF: 13.5 |
| Zhang et al.[10] | Prospective Randomized Controlled Multicenter |
111 | TDR: 44.8 (18-68) ACDF: 46.7 (18-68) |
48 | Mobi-C®(n=55) | Cage and plate (n=56) | TDR: 14.2 ACDF: 14.0 |
TDR: 19.60 ACDF: 20.10 |
TDR: 1.4 ACDF: 1.6 |
|
| Skeppholm et al.[11] | Prospective Randomized Controlled Multicenter |
153 | TDR: 46.7 (± 6.7) ACDF: 47.0 (±6.9) |
24 | Discover® (n=81) | Iliac crest bone graft (n=70) | - | TDR: 39.1 ACDF: 40.1 |
TDR: 27.4 ACDF: 28.6 |
TDR: 20.7 ACDF: 20.3 |
Table 1: Study overview.
Quality of studies
All eleven studies were prospective, randomized and controlled trials. Nine out of the the eleven studies were multicenter studies. The major baseline characteristics of the patients in each study (inclusion and exclusion criteria, number of patients, demographics, clinical data) were similar. Thus, the studies were of relatively high quality.
Clinical outcome
The mean values for the NDI scores were 20.98 (SD 9.59) in the TDR group and 22.71 (SD 8.18) in the ACDF group (Figure 1). There was no significant difference in the NDI scores between the two groups (MD 1.73; 95% CI: 6.65 to 10.10; p=0.670).
The mean VAS neck score was lower in the TDR group (20.59; SD 10.39) than in the ACDF group (23.78; SD 9.30; Figure 2) but there was no significant difference between the two groups (MD 3.19; 95% CI: 5.58 to 11.97; p=0.457).
The mean VAS arm scores were similar with 18.33 (SD 9.07) in the TDR group and 19.98 (SD 8.12) in the ACDF group (Figure 3). There was also no significant difference between the two groups in the VAS arm scores (MD 1.65; 95% CI: 6.96 to 10.26; p=0.690).
Only one study used the JOA score for the assessemt of clinical outcome. Thus, this parameter could not be used for statistical analysis.
Discussion
Up to date numerous prospective, randomized and controlled studies have been published that compare the clinical outcome two or more years after surgical treatment of cervical radiculopathy by either ACDF or TDR [7-17]. Thus, a decided analysis of higher case numbers with a conclusion about the clinical benefit of the relatively expensive cervical prostheses has become possible.
The statistical analysis of the NDI as well as the VAS for arm and neck pain after a follow-up of at least 24 months did not show any significant difference between ACDF and TDR. This result is congruent to some of the studies that were included into the meta-analysis [8,9,11,15,17]. Given the large SDs the statistical power of the analysis is not high, and thus in some of the included studies significant improvements were found for TDR of the kind one would expect [7,12-14,16]. And in some of the included studies there was at least significant proof of a better range of motion and/or less degeneration of adjacent levels in the long-term follow-up as an advantage of TDR [8,10,11,14,15]. Another meta-analysis by Zhang et al. [18] found significant fewer adverse events and fewer secondary surgical procedures.
As a consequence of our analysis the comparison of ACDF and TDR shows morphological benefits in the TDR group but an equal clinical outcome at the same time. These results have to be taken into account when indicating the implantation of a cervical disc prosthesis because the costs of such an implant are between five to ten times higher compared to a cervical cage. In the authors´ opinion is crucial to indicate TDR only in younger patients with a soft-disc herniation because the verified advantages of TDR (preservation of motion and avoidance of adjacent level degeneration) are primarly morphological benefits that might help to avoid secondary surgery of adjacent levels as it was shown by Zhang et al. [18]. These facts should be included when giving advises to the patient. Probably it would be helpful if the industry would produce cervical disc prostheses at a significantly lower price to achieve a higher number of patients being treated with TDR to get more experiences with these implants.
References
- Ding D, Shaffrey ME (2012) Cervical disk arthroplasty: Patient selection. Clin Neurosurg 59: 91-97.
- Richards O, Choi D, Timothy J (2012) Cervical arthroplasty: The beginning, the middle, the end? Br J Neurosurg 26: 2-6.
- Tharin S, Benzel EC (2012) Cervical spine arthroplasty: Fact or fiction: The absence of need for arthroplasty. Clin Neurosurg 59: 82-90.
- Griva F, Pretti F, Fazio M (2014) Does cervical arthroplasty reduce the rate of adjacent segment disease? J Neurosurg Sci 58: 41-44.
- Karabag H, Cakmak E, Celik B, Iplikcioglu AC, Soran AF (2014) Arthroplasty versus fusion for single-level cervical disc disease. JPMA The Journal of the Pakistan Medical Association 64: 1348-1351
- Shriver MF, Lubelski D, Sharma AM, Steinmetz MP, Benzel EC, et al. (2015) Adjacent segment degeneration and disease following cervical arthroplasty: A systematic review and meta-analysis. The Spine Journal: Official Journal of the North American Spine Society.
- Sasso RC, Smucker JD, Hacker RJ, Heller JG (2007) Clinical outcomes of BRYAN cervical disc arthroplasty: A prospective, randomized, controlled, multicenter trial with 24 month follow-up. Journal of Spinal Disorders & Techniques 20: 481-491.
- Murrey D, Janssen M, Delamarter R, Goldstein J, Zigler J, et al. (2009) Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. The Spine Journal: Official Journal of the North American Spine Society 9: 275-286.
- Garrido BJ, Taha TA, Sasso RC (2010) Clinical outcomes of Bryan cervical disc arthroplasty a prospective, randomized, controlled, single site trial with 48 month follow-up. Journal of Spinal Disorders & Techniques 23: 367-371.
- Coric D, Nunley PD, Guyer RD, Musante D, Carmody CN, et al. (2011) Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex|C artificial disc investigational device exemption study with a minimum 2 year follow-up: Clinical article. J Neurosurg Spine 15: 348-358.
- Zhang X, Zhang X, Chen C, Zhang Y, Wang Z, et al. (2012) Randomized, controlled, multicenter, clinical trial comparing BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion in China. Spine 37: 433-438.
- Phillips FM, Lee JY, Geisler FH, Cappuccino A, Chaput CD, et al. (2013) A prospective, randomized, controlled clinical investigation comparing PCM cervical disc arthroplasty with anterior cervical discectomy and fusion. 2 year results from the US FDA IDE clinical trial. Spine 38: E907-918.
- Vaccaro A, Beutler W, Peppelman W, Marzluff JM, Highsmith J, et al. (2013) Clinical outcomes with selectively constrained SECURE-C cervical disc arthroplasty: Two year results from a prospective, randomized, controlled, multicenter investigational device exemption study. Spine 38: 2227-2239.
- Burkus JK, Traynelis VC, Haid RW Jr, Mummaneni PV (2014) Clinical and radiographic analysis of an artificial cervical disc: 7 year follow-up from the prestige prospective randomized controlled clinical trial: Clinical article. Journal of neurosurgery Spine 21: 516-528.
- Hisey MS, Bae HW, Davis R, Gaede S, Hoffman G, et al. (2014) Multi-center, prospective, randomized, controlled investigational device exemption clinical trial comparing Mobi-C Cervical Artificial Disc to anterior discectomy and fusion in the treatment of symptomatic degenerative disc disease in the cervical spine. International journal of spine surgery 8.
- Zhang HX, Shao YD, Chen Y, Hou Y, Cheng L, et al. (2014) A prospective, randomised, controlled multicentre study comparing cervical disc replacement with anterior cervical decompression and fusion. International orthopaedics 38: 2533-2541.
- Skeppholm M, Lindgren L, Henriques T, Vavruch L, Lofgren H, et al. (2015) The discover artificial disc replacement versus fusion in cervical radiculopathy--a randomized controlled outcome trial with 2-year follow-up. The Spine Journal: Official Journal of the North American Spine Society 15: 1284-1294.
- Zhang Y, Liang C, Tao Y, Zhou X, Li H, et al. (2015) Cervical total disc replacement is superior to anterior cervical decompression and fusion: A meta-analysis of prospective randomized controlled trials. PLoS One 10: e0117826
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences