Keywords
|
| Immune checkpoint; Glioma; Targeted therapy; Glioma stem cells |
Introduction
|
| High-grade gliomas (HGG) remain one of the greatest challenges for cancer management, because current standard treatment has only achieved modest improvement of survival [1]. |
| Despite improvements in surgical technique, radiation therapy and options for systemic cytotoxic therapy, the median survival for patients with newly diagnosed glioblastoma multiforme remains poor at 15 months with trimodality therapy [2]. |
| Temozolomide (TMZ) remains the standard first-line treatment regimen for HGG despite the fact that more than 90% of recurrent gliomas do not respond to TMZ after repeated exposure[3]. |
| Glioma stem cells (GSC), isolated from human glioma tissue by their high-level expression of stem cell markers, such as CD133, exhibit a pronounced malignant potential when injected into immunecompromised mice [4]. Subsequently, in vitro cultured GSC provide a reservoir of cells that maintains the tumor by generating differentiated non-stem tumor cells. Preclinical, as well as clinical studies show that GSC are responsible for recurrences after chemo-, radio- and surgical therapy [2,5-7]. |
| Neural stem cells and GSC share several common traits, such as sustained proliferation through asymmetric divisions and a highly efficient migratory capacity in the brain. There are also similarities between the neurogenic niche where adult neural stem cells reside, and the tumorigenic niche [8,9]. Recently genome-wide transcriptional analysis identified two mutually exclusive GSC subtypes with distinct dysregulated signaling and metabolic pathways. Analysis of genetic profiles and phenotypic assays distinguished between a proneural and a mesenchymal GSC. Mesenchymal GSC display more aggressive phenotypes both in vitro and in vivo and are markedly more resistant to radiation than proneural GSC [10]. |
| Evidence from preclinical studies employing patient-derived GBM cell lines suggests that GSC therapy-resistance is caused by the concomitant activation of multiple survival pathways and the presence of drug transporter genes that enable GSC to survive standard cytotoxic therapy [11–13]. It is also reported that GCS have the ability to remain in a quiescent state during chemotherapy resulting in very low number of chemotherapyinduced apoptosis and resuming proliferation after drug removal [14]. Resistance to Temozolomide (TMZ) in glioblastomas (GBM) is reported to occur due to a 32 to 56-fold increased expression of the DNA repair enzyme O6-methylguanine DNA methyltransferase (MGMT), which is increased in GSC compared to non-GSC from the same tumor [15]. These observations combined have led to the hypothesis that a large proportion of GSC may survive TMZ therapy, that ultimately gives rise to GBM recurrence. Immunofluorescence staining of human paired primary and recurrent GBM tissue showed that GSC are found in recurrent GBM at higher frequencies compared to their untreated progenitors (Figure 1). |
| While elimination of the GSC population is regarded as a key to successful treatment of cancer, the high resistance of GSC to conventional therapy remains a therapeutic challenge [15]. |
| Recent preclinical studies suggest that glioma therapy using immune checkpoint inhibitors as monotherapy or in combination with various other therapies achieves unprecedented anti-glioma efficacy that translates into significantly prolonged survival [16]. Immunotherapy for gliomas represents one of the most promising strategies to overcome GSC resistance. |
| In the hereby-presented systematic review the authors summarize the studies assessing the anti-glioma efficacy of immune checkpoint inhibitors in preclinical studies and discuss the impact of this therapy on GSCs. |
Search Methods
|
| We selectively searched the PubMed database (https://www. ncbi.nlm.nih.gov/pubmed) for articles using the search terms “immune checkpoint and glioma”, “PD-L1 and glioma” and “CTLA-4 and glioma” employing the online search tool from the Endnote X7.2® citation program for Mac. All articles published until September 23, 2015 were included. The study authors carefully reviewed all full-text versions of the retrieved articles and summarized the studies characteristics and main findings in the presented systematic review. Articles without available fulltext versions in English were excluded. |
| The authors state that no explicit review protocol was written and published and no funding or similar support has been received for conduction of this systematic review. |
Review
|
| Gliomas induce immunesuppression |
| Glioma cells express and secrete several immunosuppressive molecules that regulate immune cell functions [17,18]. Therefore the central nervous system was considered suboptimal for sufficient antitumor immune responses, until recently. One of several glioma-induced immune compromising mechanisms includes the operation of immune checkpoints. The exact mechanisms of immunosuppression are only now being elucidated, but clearly involve a combination of factors including regulatory T cells (Tregs), tumor-associated PD-L1 expression, and CTLA-4 signaling [19] |
| Table 1 summarizes immune checkpoints and other regulators of intratumoral immune cells that have been pharmacologically controlled in preclinical glioma models to overcome gliomainduced immune suppression and to ultimately exert antiglioma effects. Their physiological function is also described briefly in Table 1. While immune checkpoints help to prevent autoimmunity under physiological conditions, gliomas actively employ these mechanisms to evade immunological attacks. Immune checkpoints are frequently activated by glioma cells and exert their immune suppressive effects by direct suppression of the activity and number of glioma-attacking immune cells and indirectly by increasing the activity and number of immunosuppressive glioma-infiltrating Tregs and myeloid derived suppressor cells (MDSC). High-grade glioma patients suffer global compromise of their cellular immunity, characterized by dramatic reductions in CD4+ T cell numbers and function. It has been shown that this is attributed to an increased number of glioma-infiltrating Tregs [20]. Normally Tregs control tolerance of self-antigens by suppressing autoimmunity, while also enabling effective immune responses towards non-selfantigens. In GBM Tregs contribute to immunotherapeutic failure, ultimately leading to tumor progression [21]. The subpopulation of Tregs (CD4+CD25+Foxp3+) constitutes 5%-10% of CD4+ cells and plays a crucial role in suppressing anti-glioma immune response. The number of Tregs is significantly higher in patients with glioblastoma multiforme than in healthy controls and is inversely correlated with the frequency of glioma-infiltrating CD3+ activated T-cells. This may suggest that Tregs represent an important target for immunotherapy [22-24]. |
| High-grade glioma (HGG) patients exhibit high expression levels of immune checkpoint-activating molecules within the tumor tissue and in serum. For example, CD200, a positive regulator of MDSC [25], and the immunesuppressive Fgl2 (Fibrinogen-like protein 2), are highly expressed in tumor tissue and serum. Fgl2 mRNA is significantly higher expressed in high-grade gliomas compared to low-grade gliomas; higher expression levels are therefore associated with shorter overall survival [26]. The clinically most important immune checkpoints PDL-1 (Programmed cell death protein 1 ligand), IDO (Indoleamine 2,3-dioxygenase) and CTLA- 4 (Cytotoxic T-lymphocyte-associated protein 4) are also highly expressed in GBM and tightly correlated with survival [17,24]. Several other immune regulatory molecules have been described to be of diagnostic and prognostic value and have been recently proposed as promising targets for immunotherapy of HGGs [27,28]. |
| Immunechekpoint inhibition |
| Immunotherapeutic strategies for the treatment of gliomas include the use of autologous stimulated lymphocytes, immunotherapy with cytokines and dendritic cells, immune checkpoint inhibitors, virotherapy, and tumor or peptide based vaccines and are currently under active investigation [29]. |
| Especially immune checkpoint blockade with anti-CTLA-4 and anti-PD-1 have demonstrated encouraging results in clinical trials with other solid tumors and are yet to be performed for gliomas. Recent data suggest that this type of therapy may be particularly useful for tumors with high mutational burdens, which is the case in HGGs [30]. Therefore, it is thought that particularly immune checkpoint inhibitors will play a crucial role in immunotherapeutic approaches against HGGs [31]. |
| Immune checkpoint molecules like PD-1, CTLA-4, and the T cell inhibitor TIM3 act as negative regulators of the immune system and are upregulated in GBM [32,33]. Their immunosuppressive, glioma-propagating effects are substantially caused by their ability to activate and increase the frequency of intratumoral Tregs. The suppressive activity of Tregs has been implicated as an important factor limiting immune mediated destruction of tumor cells. Given the potently immunosuppressive function of Tregs, immune checkpoint inhibition is thought to inactivate Tregs, while simultaneously reactivating the cytotoxic lymphocyte response [34]. |
| Table 2 summarizes the most important characteristics and main findings of preclinical studies that evaluated the anti-glioma efficacy of immune checkpoint inhibition in murine glioma models. |
| With few exceptions, immune checkpoint inhibitors administered as monotherapy have been reported to show little or no improvement of survival in preclinical trials. Whereas anti-CTLA-4 monotherapy lead to a survival advantage highly significant with 80% survival at day 100 in a study by Fecci et al. [20] and 50% survival at day 90 after tumor inoculation in a study by Grauer et al. [23], other authors report a significant effect only in early tumor stages [35] or no advantage in survival at all [36]. The variable response to anti-CTLA therapy is thought to be attributed to a time-dependent upregulation of CD25, CTLA-4, and other immune checkpoints on intratumoral Tregs during tumor growth [23]. |
| PD-1 blockade has been uniformly reported to result in no survival benefit when administered as monotherapy [32,33,37]. Both, CTLA-4 and PD-1 blockade as well as anti-CD25 therapy exert their anti-glioma effects by reducing the number of highly suppressive Tregs within the growing tumor and provoking a CD4 and CD8 T cell dependent destruction of the glioma cells. Accordingly, anti-CD25 monotherapy results in similar survival advantages like anti-CTLA-4 monotherapy [23,38]. |
| The immune inhibitory molecules IDO and Fgl2 modulate the glioma-induced immunesuppression on a broader basis. Fgl2 generates and activates not only Tregs, but also MDSC and macrophages. Additionally to that, IDO physiologically suppresses T cells and NK cells. Hence, IDO blockage, as well as Fgl2 blockage lead to significant survival advantages over controls when administered as monotherapy [26]. This suggests that Fgl2 and IDO function as key immune-suppressive modulators and have potential as an immunotherapeutic target for treating GBM. |
| However, selective targeting of one component of a dysregulated pathway may be inadequate for a durable clinical response, given the intratumoral heterogeneity of GBM and hypermutated profiles displayed by tumor recurrences. Accordingly, in most studies the anti-glioma effects of combinations of two to three agents and radiotherapy have been assessed (Table 2). Whereas PD-1 blockage is ineffective as monotherapy the combination of PD-1 blockage and stereotactic radiotherapy results in a synergistic anti-glioma effect and long-term survivors [33,37]. Given that tumor-infiltrating lymphocytes can express multiple checkpoints and expression of 2 or more checkpoints corresponds to a more exhausted T-cell phenotype, Kim et al. [33] added TIM-3 blockage to PD-1 blockage and stereotactic radiotherapy, which resulted in a significant improvement in survival compared with single and double treatment with an overall survival of 100% by day 146. Also CTLA-4 blockage has been shown to be much more effective when combined with radiotherapy, with 40% survival of day 90 after tumor inoculation [39]. |
| Noteworthy, Belcaid et al. [39] investigated the impact of timing of treatment start in relation to tumor cell inoculation on survival. Hence, three cohorts receiving radiotherapy on day 10 and starting with anti-CTLA-4 therapy on day 8, 10 and day 12, respectively, following tumor inoculation have been studied. Although the authors found no significant difference in survival, a trend is revealed of longer survival with earlier timing of anti-CTLA-4 therapy. Wainwright et al. [16], started triple immune checkpoint inhibition with CTLA-4 blockage on day 3 and day 7 post-inoculation and this resulted in 100% survival and 78% survival at day 90. Moreover, Agarwalla et al. [35] found significant anti-glioma efficacy of CTLA-4 blockage in the cohort that received treatment early after tumor inoculation (day 3), but not in the cohort starting treatment on day 12. Anti-CTLA-4 monotherapy starting as late as day 22 after tumor inoculation resulted in no significant survival advantage at all [36]. Table 2 specifies the treatment starts after tumor inoculation for all treatment methods in reports studying immune checkpoint blockade in glioma models. The previously described preclinical data suggest that immune checkpoint inhibiton is efficacious only when tumor burden is very low. Translated into clinical situation this data suggest that immune checkpoint inhibition might only exert significant anti-glioma efficacy when administered after total/subtotal resection or in combination with multiple pathway blockage or other treatment modalities, e.g., radiotherapy. |
| Whereas reported long-term surival using multiple immune checkpoint blockages implicates considerable effects on GCS and therapy-resistance, the issue is rarely addressed experimentally in the existing studies. Huang et al. [40] studied the effects of NK therapy alone and PD-1 inhibited NK therapy on a stem cell enriched glioma cell line in vitro and GSC-enriched xenografts in vivo. Interestingly they found a significantly lower PD-L1 expression on GSC compared to the non-GSC differentiated cells. They hypothesized that GSC-resistance could be overcome by adding activated NK to the regimen, that have been reported to be effective against GSC. They demonstrated that inhibition of the PD-1/B7H1 pathway promotes the co-toxicity of NK cells against GSCs in vitro and in the intracranial GSCs model, mice that received PD-1-inhibited NK treatment showed longer survival and slower tumor growth.Recent evidence revealed that GSCs contribute more to tumor development than non-GSCs by a more pronounced attenuation of immune surveillance. GSCs express or secrete immune-suppressive factors that locally suppress the immune response. CD133+ glioblastoma cell populations secrete more TGFβ than CD133- glioblastoma cell populations [41]. This leads to a expansion of Treg population and attenuation of MHCII expression [42, 43]. Additionally GSCs are able to recruit tumor associated macrophages that support tumorprogression by enhancing immune-supressive microglia phenotypes [44]. Given that the GSC population especially exploits immune-suppression to promote tumor growth, abrogation of glioma-induced immunesuppression with immune checkpoint inhibitors seems to be a promising rationale to enhance anti-GSC efficacy. |
Conclusion
|
| Immunotherapy with immune checkpoint inhibitors is coming to the fore as a viable anti-cancer treatment modality, even in poorly immunogenic cancers such as GBM. Current data suggests that chemoradiation may not preclude the success of immunotherapeutics, as their effects may be synergistic. |
| Although these studies show a high anti-glioma efficacy of immune checkpoint inhibitors, especially when multiple pathways are inhibited, their impact on GSC is rarely addressed and the mechanisms behind it are far from fully elucidated. Nevertheless, recent evidence revealed that GSCs contribute more to tumor development than non- GSCs by a more pronounced attenuation of immune surveillance. Therefore abrogation of glioma-induced immunesuppression with immune checkpoint inhibitors seems to be a promising rationale to enhance anti-GSC efficacy. |
| Clinical trials studying immune checkpoint blockage in high-grade gliomas have been recently designed with some of them already enrolling patients. |
Acknowlegment
|
| The authors would like to thank Martin Oft MD for critically revising the manuscript and his thoughtful comments regarding the contents of this work. |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
Figures at a glance
|
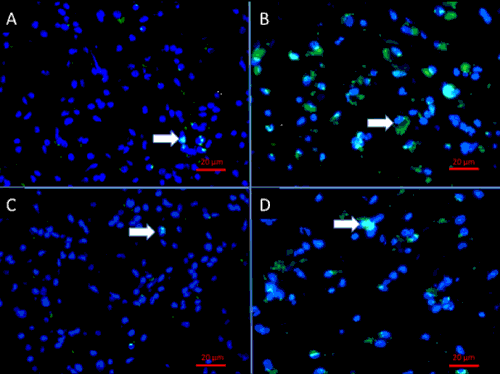 |
| Figure 1 |
|
References
|
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987-996.
- Florian IS, Tomuleasa C, Soritau O, Timis T, Ioani H, et al. (2011) Cancer stem cells and malignant gliomas. From pathophysiology to targeted molecular therapy. J BUON 16: 16-23.
- Chong DQ, Toh XY, Ho IA, Sia KC, Newman JPet al. (2015) Combined treatment of Nimotuzumab and rapamycin is effective against temozolomide-resistant human gliomas regardless of the EGFR mutation status. BMC Cancer 15:255.
- Singh SK, Clarke ID, Hide T, Dirks PB (2004) Cancer stem cells in nervous system tumors. Oncogene 23: 7267-7273.
- Kim SH, Kwon CH, Nakano I (2014) Detoxification of oxidative stress in glioma stem cells: mechanism, clinical relevance, and therapeutic development. J Neurosci Res 92: 1419-1424.
- Hira V V, Ploegmakers KJ, Grevers F, Verbovsek U, Silvestre-Roig C, et al. (2015) CD133+ and Nestin+ Glioma Stem-Like Cells Reside Around CD31+ Arterioles in Niches that Express SDF-1alpha, CXCR4, Osteopontin and Cathepsin K. J HistochemCytochem 63:481–493.
- Chen J, Li Y, Yu TS, McKay RM, Burns DK, et al. (2012) A restricted cell population propagates glioblastoma growth after chemotherapy. Nature488:522–526.
- Lindberg N, Jiang Y, Xie Y, Bolouri H, Kastemar M, et al. (2014) Oncogenic signaling is dominant to cell of origin and dictates astrocytic or oligodendroglial tumor development from oligodendrocyte precursor cells. J Neurosci 34: 14644-14651.
- Xiong A, Kundu S, Forsberg-Nilsson K (2014) Heparan sulfate in the regulation of neural differentiation and glioma development. FEBS J 281: 4993-5008.
- Nakano I 2015 Stem cell signature in glioblastoma: therapeutic development for a moving target. J Neurosurg 122: 324-330.
- Signore M, Pelacchi F, di Martino S, Runci D, Biffoni M, et al. (2014) Combined PDK and CHK inhibition is required to kill glioblastoma stem-like cells in vitro and in vivo. Cell Death Dis 5: e1223.
- Mehta M, Khan A, Danish S, Haffty BG, Sabaawy HE (2015) Radiosensitization of Primary Human Glioblastoma Stem-like Cells with Low-Dose AKT Inhibition. Mol Cancer Ther 14: 1171-1180.
- Rizzo AE, Yu JS (2015) Radiation therapy for glioma stem cells. AdvExp Med Biol 853: 85-110.
- Annovazzi L, Caldera V, Mellai M, Riganti C, Battaglia L, et al. (2015) The DNA damage/repair cascade in glioblastoma cell lines after chemotherapeutic agent treatment. Int J Oncol46:2299–308.
- Okada M, Sato A, Shibuya K, Watanabe E, Seino S, et al. (2014) JNK contributes to temozolomide resistance of stem-like glioblastoma cells via regulation of MGMT expression. Int J Oncol 44: 591-599.
- Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim CK, et al. (2014) Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res 20: 5290-5301.
- Avril T, Saikali S, Vauleon E, Jary A, Hamlat A, et al. (2010) Distinct effects of human glioblastomaimmunoregulatory molecules programmed cell death ligand-1 (PDL-1) and indoleamine 2,3-dioxygenase (IDO) on tumour-specific T cell functions. J Neuroimmunol225:22–33.
- Perng P, Lim M (2015) Immunosuppressive Mechanisms of Malignant Gliomas: Parallels at Non-CNS Sites. Front Oncol 5: 153.
- Bloch O (2015) Immunotherapy for malignant gliomas. Cancer Treat Res 163: 143-158.
- Fecci PE, Ochiai H, Mitchell DA, Grossi PM, Sweeney AE, et al. (2007) Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res13:2158–2167.
- Wainwright DA, Dey M, Chang A, Lesniak MS (2013) Targeting Tregs in Malignant Brain Cancer: Overcoming IDO. Front Immunol4:116.
- El Andaloussi A, Lesniak MS (2006) An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastomamultiforme. NeuroOncol. 8:234–243.
- Grauer OM, Nierkens S, Bennink E, Toonen LW, Boon L, et al. (2007) CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer121:95–105.
- Kmiecik J, Poli A, Brons NH, Waha A, Eide GE, et al. (2013) Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol 264:71–83.
- Moertel CL, Xia J, LaRue R, Waldron NN, Andersen BM, et al. (2014) CD200 in CNS tumor-induced immunosuppression: the role for CD200 pathway blockade in targeted immunotherapy. J Immunother Cancer 2:46.
- Yan J, Kong LY, Hu J, Gabrusiewicz K, Dibra D, et al. (2015) FGL2 as a Multimodality Regulator of Tumor-Mediated Immune Suppression and Therapeutic Target in Gliomas. J Natl Cancer Inst 107.
- Fong B, Jin R, Wang X, Safaee M, Lisiero DN, et al. (2012) Monitoring of regulatory T cell frequencies and expression of CTLA-4 on T cells, before and after DC vaccination, can predict survival in GBM patients. PLoS One 7: e32614.
- Liang H, Yi L, Wang X, Zhou C, Xu L (2014) Interleukin-17 facilitates the immune suppressor capacity of high-grade glioma-derived CD4 (+) CD25 (+) Foxp3 (+) T cells via releasing transforming growth factor beta. Scand J Immunol 80:144–150.
- Weathers SP, Gilbert MR (2015) Current challenges in designing GBM trials for immunotherapy. J Neurooncol 123: 331-337.
- Wang JY, Bettegowda C (2015) Genetics and immunotherapy: using the genetic landscape of gliomas to inform management strategies. J Neurooncol 123: 373-383.
- Karlitepe A, Ozalp O, Avci CB (2015) New approaches for cancer immunotherapy. TumourBiol 36: 4075-4078.
- Wainwright D a, Chang a L, Dey M, Balyasnikova I V, Kim CK, et al. (2014) Durable Therapeutic Efficacy Utilizing Combinatorial Blockade against IDO, CTLA-4, and PD-L1 in Mice with Brain Tumors. Clin Cancer Res. 20:5290–5301.
- Kim JE, Patel MA, Mangraviti A, Velarde E, Theodros D, Mathios D, et al. (2015) 143 The Combination of anti-TIM-3 and anti-PD-1 Checkpoint Inhibitors With Focused Radiation Resulted in a Synergistic Antitumor Immune Response in a Preclinical Glioma Model. Neurosurgery 1:212.
- Wainwright DA, Lesniak MS (2014) Menage a trois: Sustained therapeutic anti-tumor immunity requires multiple partners in malignant glioma. Oncoimmunology3:e28927.
- Agarwalla P, Barnard Z, Fecci P, Dranoff G, Curry Jr. WT (2012) Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J Immunother35:385–389.
- Vom Berg J, Vrohlings M, Haller S, Haimovici A, Kulig P, et al. (2013) Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J Exp Med 210: 2803-2811.
- Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, et al. (2013) Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J RadiatOncolBiolPhys 86: 343-349.
- El Andaloussi A, Han Y, Lesniak MS (2006) Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg. 105:430–437 A
- Belcaid Z, Phallen JA, Zeng J, See AP, Mathios D, Gottschalk C, et al. (2014) Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One 9:e101764.
- Huang BY, Zhan YP, Zong WJ, Yu CJ, Li JF, et al. (2015) The PD-1/B7-H1 pathway modulates the natural killer cells versus mouse glioma stem cells. PLoS One 10: e0134715.
- Lottaz C, Beier D, Meyer K, Kumar P, Hermann A, et al. (2010) Transcriptional profiles of CD133+ and CD133- glioblastoma-derived cancer stem cell lines suggest different cells of origin. Cancer Res 70:2030–2040.
- Facoetti A, Nano R, Zelini P, Morbini P, Benericetti E, et al. (2005) Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res. 11:8304–8311.
- Wei J, Barr J, Kong LY, Wang Y, Wu A, et al. (2010) Glioma-associated cancer-initiating cells induce immunosuppression. Clin Cancer Res 16: 461-473.
- Wu A, Wei J, Kong LY, Wang Y, Priebe W, et al. (2010) Glioma cancer stem cells induce immunosuppressive macrophages/microglia.NeuroOncol 12: 1113-1125.
|

