Middle Meningeal Artery Embolization as a Treatment for Chronic Subdural Hematomas: A Literature Review
Ali Msheik*
Department of Neurosurgery, Dr. Rajendra Prasad Government Medical College (RPGMC), Himachal Pradesh, India
- *Corresponding Author:
- Ali Msheik
Department of Neurosurgery,
Dr. Rajendra Prasad Government Medical College (RPGMC),
Himachal Pradesh,
India,
Tel: 0096170762594;
E-mail: Dr.alimsheik@gmail.com
Received date: December 01, 2022, Manuscript No. IPJNCS-22-15096; Editor assigned date: December 05, 2022, PreQC No. IPJNCS-22-15096 (PQ); Reviewed date: December 20, 2022, QC No. IPJNCS-22-15096; Revised date: January 20, 2023, Manuscript No. IPJNCS-22-15096 (R); Published date: January 27, 2023, DOI: 10.36648/2471-9633.7.1.002
Citation: Msheik A (2023) Middle Meningeal Artery Embolization as a Treatment for Chronic Subdural Hematomas: A Literature Review. Neurosurg Vol:7 No:1
Abstract
Background and objective: This is a literature review aiming to provide an update about the recent findings related to efficacy of MMA embolization in the treatment of chronic subdural hematomas, comparison with conventional therapy and deduction of the current recommendations and indications.
Subjects and methods: The literature is reviewed using a search through the PubMed index using keywords. Studies are then screening, skimmed and thoroughly read. 32 studies ultimately fulfilled the inclusion criteria and are included.
Results: Five indications as for the application of MMAE are deducted from the literature. The usage for as a preventive measure after surgical treatment of symptomatic cSDHs in patients with high risk of recurrence and the usage as a standalone procedure have been the most common reasons for indication of this procedure. Rates of failures for the aforementioned indications have been 6.8% and 3.8% respectively.
Conclusion: Safety of MMAE as a procedure is regarded as a general theme through the literature and can be considered for future application. Usage of this procedure in clinical trials with more patient segregation and time frame assessment relative to surgical intervention are recommendations of this literature review.
Keywords
cSDHs; MMAE; Burr hole; Recurrence; Prevention; Elderly population; Inflammation
Abbrevations:
cSDH: chronic Subdural Hematoma; MMA: Middle Meningeal Artery; MMAE: Middle Meningeal Artery Embolization; MRI: Magnetic Resonance Imaging; CT scan: Computed Tomography scan; CSF: Cerebro Spinal Fluid
Introduction
Definition of subdural hematoma
Chronic Subdural Hematoma (CSDH) is defined as a liquefied hematoma in the subdural space [1]. A cSDH has a characteristic outer membrane that can be up to 10 mm in thickness [2]. It is usually linked to a recent incident of cranial trauma [3]. CSDH appears as a crescentic mass with hypodense to isodense consistency on cranial computed tomography. It requires clinical and radiological experience to discern the difference among cSDHs and hygromas [4]. A subdural hygroma contains Cerebrospinal Fluid (CSF) and bleeding could have occurred at some point Hence, it is termed as xanthochromic. Dehiscence of the subdural space and the arachnoid space dictates a spectral variance of cSDHs from effusions to hygromas and eventually actual bleeding cSDHs. The latter consist due to the acuity of the bleeding a separate spectrum of cSDHs [5].
Anatomy and histology
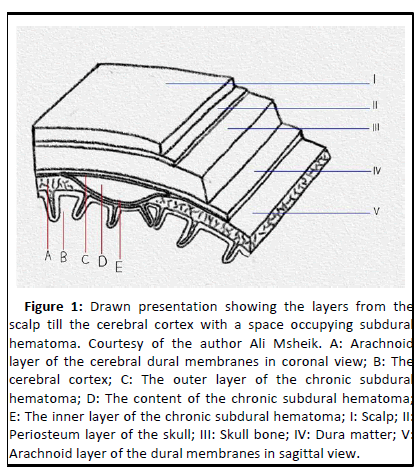
CSDHs are encapsulated flattened structures that form within the virtual dural space (Figure 1). In 19% of the case, cSDHs are bilateral [6]. The median fluid volume in cSDHs was 96 mL (range 33 mL-270 mL) in 28 patients operated on in one series and 93 mL (range 20 mL-170 mL) in another series of 19 patients with 22 cSDHs [7]. The aforementioned outer membrane of cSDHs closely resembles the granulation tissue that develops after injury to the dermis. Neoangiogenesis from dural capillaries and proliferation of marginal cells of the dural layer lead to formation of the outer cSDH layer and promotes bleeding [8]. Histologically, this membrane comprises fibroblasts, myofibroblasts, mast cells and eosinophils. Richness in thin walled sinusoidal blood vessels is evident along with smooth muscles cells and is reported. With a diameter of 80 micrometers, endothelial fenestrations, gap junctions, and an incomplete basement membrane and pericyte layer, those vessels are susceptible to spontaneously bleed or secondary to inflammatory and fibrinolytic activity in the cSDH fluid. Evidence of erythropoiesis in the extracellular space as erythrocytes and in the cSDHs outer membranes as erythroblasts is reported [9].
Figure 1: Drawn presentation showing the layers from the scalp till the cerebral cortex with a space occupying subdural hematoma. Courtesy of the author Ali Msheik. A: Arachnoid layer of the cerebral dural membranes in coronal view; B: The cerebral cortex; C: The outer layer of the chronic subdural hematoma; D: The content of the chronic subdural hematoma; E: The inner layer of the chronic subdural hematoma; I: Scalp; II: Periosteum layer of the skull; III: Skull bone; IV: Dura matter; V: Arachnoid layer of the dural membranes in sagittal view.
Evolution
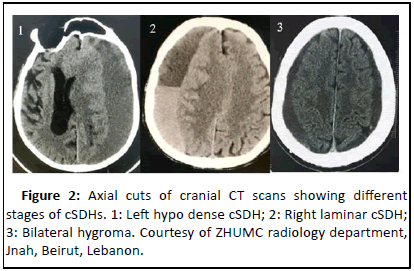
Being of embryonic mesodermal origin, tear of the cell layer of the meninges dictates a similar response to wound healing [10]. Hemorrhage promotes platelets activation and the subsequent steps of the coagulation cascade. Attempts to stage cSDHs according to levels of factors secreted and implemented in the coagulation cascade have failed due to the recurrent cycles of bleeding within a cSDH [11]. As one coagulation and fibrinolysis cycle is progressing, another cycle can start and variations within the same cSDH histologically and immunochemically can manifest. When the initial bleeding starts, hemolysis pursues. The inner layer of the cSDH, which is the initial dural layer, is mainly avascular. The subsequent formation of granulation tissue, in the presence of platelets growth factors, allows the formation a dural outer layer. Neovascularization promotes vascular supply to this layer. tPA leads to fibrinolysis though plasmin activation and hence, can lead to rebleeding. The stage at which the cSDH is imaged and monitored belongs to a wide spectrum. Differentials for a hypo dense crescentic collection include subdural hygroma (discussed earlier) and subdural empyema [12]. Definitive diagnosis is mainly through MRI and clinical history. A cSDH starts as an acute sub dural hematoma [13]. Typically, on CT scan, the latter is hyper dense. If the cSDH started from a hygromas, the latter appears a homogenous low density collection of cranial CT scan. This discrepancy in intensity relates to the presence and concentration of hemoglobin, which is hyper dense on CT scans, and signifies the acuity of the hematoma and being of a sanguineous consistency. Degradation of hemoglobin over the course of time delineates the progression of the cSDH. Hence, a cSDH turns less dense with time. Rebleeding shows as a cSDH with layers of different intensities 2 of 17. Hence, it is laminar.
Complete fibrinolysis and resolution of the cSDH is termed as trabecular cSDH [14].
Correspondence of CT appearance with classification and prognosis
Postsurgical risk for recurrence and hematoma volume increase may be correlated to the CT scan features obtained before surgical intervention i.e. low risk is attributed to the homogenous appearance of cSDHs on CT scan [15]. Due to the chronicity of the SDH, ideally it may appear as hypo dense. Radiologists can miss bilateral cSDHs. However, failure of the convexity of the sulci to reach the inner table of the skull, and the medial displacement of the white matter gray matter interface are indicative of presence of cSDHs. The highest risk of progression of cSDHs is attributed to laminar and separated cSDHs (Figure 2). As previously aforementioned, the trabecular stages represent a mature stage of cSDHs and hence have a low risk of progression or rebleeding [16].
Causes of subdural hematoma
Bleeding: Tear of bridging veins as they traverse the dural cell layer is the most accepted cause of cSDHs [17]. Inertial brain injury secondary to direct trauma both major and minute is reported in two thirds of patients and can provoke tear of those fragile vessels. Support of this theory is surgical findings describing veins with clotted ends recovered after removed of the cSDHs outer layer [18].
Transformation from hygroma: Formation of a cSDH as a progression from a sub-acute hygroma is another hypothesis advocated by Japanese neurosurgeons. In the 1960’s and 1970’s, the latter presumed that trauma provoked dural cell layer tears, which led to CSF leak, which in terms promoted outer granulation tissue formation and a wound healing response [19]. Transformation into cSDHs was theorized to have been due to bleeding into the preformed hygromas due to subsequent tear of bridging vessels. Support of this theory was attained by prevalence of thin subdural neomembranes in 4% of consecutive autopsy specimen. However, this does not hinder the possibility of unreported trauma in the medical history of these patients [20].
Enlargement by osmotic and oncotic pressures: Indifference in osmolality and oncotic pressure between cSDH fluid, plasma and CSF limits the credibility of the theory supporting the role of either in the enlargement of cSDHs. On the other hand, the fact that a wound healing process along with the coagulation cascade are well-documented processes in cSDHs development, an undeniable role of transudation and exudation must be considered in the evolution of cSDHs.
Epidemiology of cSDH
The incidence of CSDH is not well known and varies between operated and non-operated cases. Ranging from 1.3 to 5.3 per 100,000 per year for operative cases, the incidence naturally increases to 8.2 up to 48 per 3 of 17 100,000 per year. Compared to results of Helsinki study by Foelfolm, et al. in 1975, there is a rough 25 times increase in incidence. This can be attributed to the advances in medical imaging, increase in the aging population, and the increasing use of anticoagulants and antiplatelet medication. This is further fortified by the fact that the mean age of diagnosis is about 75 years of age for males and 74 years of age for females. Of note, male predominance is widely reported in literature with a ratio of 2:1 up to 5:1. Factors associated with increased risk of CSDH are age, alcoholism, male gender, use of Oral Anticoagulants (OACs), neurological and systemic illnesses associated with brain atrophy, hemodialysis, and conditions associated with low intracranial pressure and dural tear.
Treatments for cSDH
Treatment of cSDH requires prior assessment of patients on an individual basis. The symptomatology of patients is key in determining the urgency and the indication for surgical intervention. Typically, patients received at the ED for trauma or acute development of neurological compromise are diagnosed or incidentally found to have cSDH. History of trauma, neurological compromise, the acuity of the symptoms, and the medical comorbidities associated with the condition of the patients of whom the elderly populations are the vast majority govern the decision making process. Respiratory diseases, cardiac sufficiency, renal compromise, old age, and need for OACs and antiplatelets medication dictate the necessity for pre surgical evaluation and preparation of patients to whom surgery is considered indicated.
Correction of coagulopathy and thrombopathy: Reversal of coagulopathy instilled by OACs and antiplatelet medications, liver insufficiency and other coagulation disease is essential for the prevention of further expansion of cSDHs and to allow control of blood loss after surgical evacuation of symptomatic cSDHs. This can be done by utilization of FFPs (Fresh Frozen Plasma) for life saving procedures and interruption of medication for few days before elective surgical evacuation.
Adjuvant treatments: Steroids and anti-epileptic medications are two main medical therapies adjuvant to surgical evacuation used in managing symptomatic cSDHs. Shrestha DB, et al. reported in 2021 lesser recurrence of cSDH under the treatment with steroids. However, no benefit of the steroid based versus non-steroidal treatment in terms of mortality and treatment success was noted. A significant increase of adverse side effects was correlated with steroid treatment i.e. hyperglycemia, hypertension, immunocompromised status. According to Nachippan DS, et al., reported incidence of seizures varies from 0.7% to 18.5% in patients with cSDHs. The authors noted lack of evidence of significant reduction in incidence of seizures in the patients with cSDHs following administration of anti-epileptic drugs.
Conservative treatment: Conservative treatment is offered to patients whom morbid conditions hinder surgical intervention or to whom surgery will not add benefit compared to medical treatment. Patients with small asymptomatic cSDHs are managed conservatively. However, no pre set guidelines are applied. Mainly OACs and anti-platelet medication are stopped along with FFPs administration and correction of coagulopathies instilled by liver insufficiency and comorbid conditions. Routine monitoring of the cSDH volume and evolution depends on the physician following the patient's condition. Some resources are pragmatic and resort to repetition of a cranial CT scan as patients develop symptoms. Other physicians consider a 2-week routine CT scan as a regimen.
Surgical intervention: Symptomatic patients are ideally treatment with surgical evacuation with 80% success rate. Accomplished with minimal surgical risk, usually patients recover fast postoperatively and show reverse of neurological compromise instilled by the expanded hematoma.
Prognosis and complications: Complications of cSDH surgical evacuation range from the ones associated to any surgery i.e. infection, bleeding and possible tissue damage to more specific ones related to the nature of the operation i.e. continuous bleeding, rebleeding, tension pneumocephalus, seizures, intraparenchymal bleeding, stroke and possible brain tissue damage. In the literature, reports of recurrence define the latter by rebleeding into an evacuated cSDH in 2.3% to 33% of cases. Factors promoting recurrence are summarized in the following: preoperative OAC and antiplatelets medication, intracranial air, poor intraoperative brain re-expansion, bilateral cSDHs, and postoperative midline shift persistence.
Mortality due to cSDHs varies according to whether a surgical intervention was held or not. Without surgical evacuation, mortality rises up to 33%. However, it drops to 3% within 30 days of cSDHs evacuation. Prognosis postoperatively depends on the preoperative morbid condition, extent of neurological symptoms 4 of 17 and the age of the patient.
Future research areas
New approaches: Surgical evacuation and brain decompression are main goals of treatment of symptomatic cSDHs. Postoperative adjuvant therapies are understudy in the literature inclusive of corticosteroids, ACE inhibitors and tranexamic acid. Evidence of lower recurrence rates are associated with corticosteroid administration. However, more clarification is foreseen for the other two modalities. Recently, an interventional approach was suggested. It is the embolization of the Middle Meningeal Artery (MMA) as means to devascularize the outer layer of the cSDH to prevent further rebleeding and hence limit recurrence.
Anatomy related to MMA embolization: It is believed that the MMA through its anterior and posterior branches, which supply the dural layers, can anastomose with the neovascular vessels. Hence, rebleeding through these anastomoses can provoke recurrence of a cSDH. Embolization of MMA's branches related the position of the cSDH is believed to limit the rebleeding and hence prevent recurrence.
Steps of MMA embolization: Embolization requires an endovascular approach through the femoral artery ipsilateral to the recurrent cSDH. Guided progression through the femoral artery through the aorta through either the left or right carotid artery terminates in the MMA branching from the maxillary branch of the external carotid artery. The desired part is embolized usually using N-Butyl-2-Cyanoacrylate (NBCA). Stasis of the contrast thereafter indicates success of the embolization.
Literature Review
Aim of the literature review
The aim of this literature review to provide an update about the recent findings related to efficacy of MMA embolization. Answers that this review will provide connect to the following inquiries:
• Is MMA embolization as adjunctive technique besides surgical intervention?
• Can MMA embolization replace surgery? In which patients?
• What are the indications for MMA embolization?
• How efficient is MMA embolization in prevention of recurrence after surgical intervention and expansion of minimal cSDH not requiring surgical evacuation?
• What does the failure of MMA embolization to prevent recurrence of cSDH rebleeding suggest?
Primary revision: Identification
We reviewed the existing literature on PubMed until August 2022, in English language. We performed the research with combinations of the keywords "MMA", "middle meningeal artery embolization", "embolization", "refractory cSDH", "chronic subdural hematoma recurrence", and "cSDH outer layer". All kinds of work were included. References of the relevant studies were considered as an additional source of articles. Pure literature reviews were not included. We focused on the cSDH and the embolization of MMA as means of treatment.
Secondary revision: Screening
The titles of the sequestered articles are screened. Causes of cSDH related to acute subdural and epidural hematomas and tumor provoked cSDHs were excluded. Studies inclusive of pure methodological description of MMAE and MMA variable anatomy were excluded.
Data sequestration: Inclusion and classification
As this literature review is work of one author, data of the included studies were thoroughly read and analyzed. Referral to other recent literature reviews aided for the guidance of the author and allowed further checking. The studies were classified according to way MMAE was utilized. Hence, the patients were classified according to the indication of MMAE:
• MMAE as a standalone therapy.
• MMAE to prevent recurrence in symptomatic cSDHs.
• MMAE in recurrent cSDHs without second surgery.
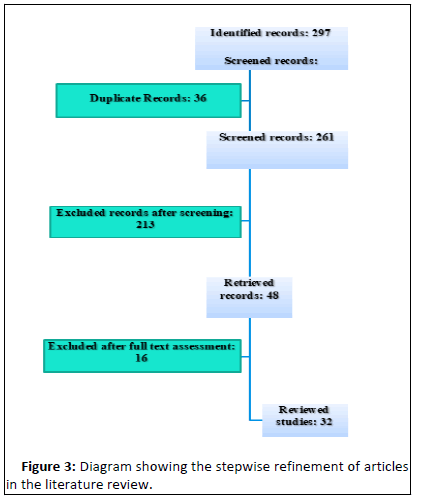
• MMAE in patients with recurrent cSDHs after second surgery (Figure 3).
Results
297 records were identified after search using the combination of the aforementioned keywords on PubMed. 36 records were exempted because of being duplicate records. 213 records were excluded after screening of the titles and abstracts. 13 records were excluded after reading the full text. Exclusion was due to the following reasons: acute hematomas (1), CSF hypotension (2), tumor associated cSDHs (3) and technical description of MMAE without patient follow up (6).
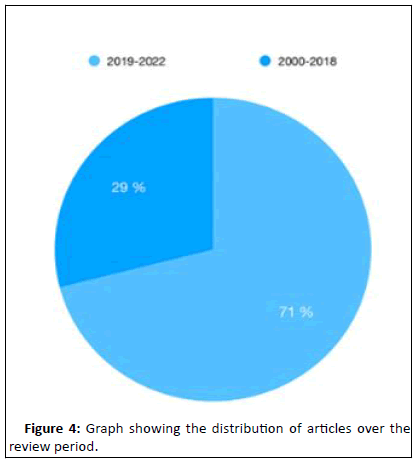
32 records were finally reviewed. A spreadsheet was established inclusive of the following details fragmenting each study. Among the 32 eligible studies, 20 were case series, 10 were case reports, one was a prospective clinical trial and another was a retrospective study. Five of the case series and the aforementioned retrospective study presented each a comparison with an historical group of patients treated conventionally. Figure 4 shows the distribution of the studies along the revision period. More than two-thirds (79%) of the studies were published after 2019.
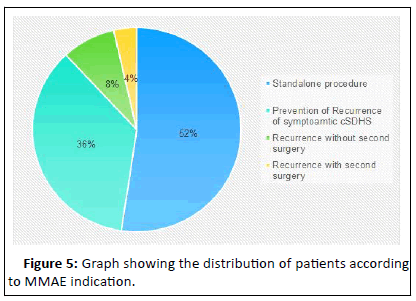
Totally, 973 patients were included and categorized into five groups. The distribution of the patients is evident in Figure 5. It is evident that more than half (exactly 53%) of the MMAE procedures were done without further surgical intervention. About one third of the MMAE procedures were done for to prevent recurrence after primary surgical intervention for symptomatic cSDHs. 82 cases of MMAE (8.4%) were done as treatment for recurrence of cSDHs another surgical intervention. Only 35 MMAEs were done after a second surgery was done for recurrence. Arham, et al. was the only author reporting MMAE application at the time of the first surgery as conjoint treatment with surgery. Of note, none of the MMAE procedures were done concurrently with or before the surgical intervention. All procedures were done either solely or after surgical evacuation of the cSDHs were done.
MMAE as a standalone procedure
Being a standalone procedure is an application of MMAE reported in 14 of the reviewed articles published starting from the year 2018 (Supplementary Table 1). Before 2018, no report of any MMAE procedures as a standalone procedure was found through this review. However, indications dictating this application have been varied through the reviewed articles. Below is enlisted for each article was MMAE was applied as a standalone procedure the corresponding indication (s). The patient's preference was the main indication for a standalone MMAE in 3 of the aforementioned articles. Okuma, et al., and Yajima, et al. proposed a standalone MMAE procedure for elderly patients on OAC and antiplatelet medication. Entezami, et al. added to this indication the application of MMAE as a standalone procedure for COVID-19 patients. Poor surgical candidacy with hematoma expansion and failed conservative treatment were reasons suggested by Mureb, et al. to back up standalone MMAE in the studied population. Of note, four studies did not include clarification concerning the indication of standalone MMAE.
MMAE as a prevention of recurrence for symptomatic cSDHs
Of the 773 patients included in the studies, 516 (66.75%) were subject to a standalone MMAE procedure with 3.8% failure rate if compared to the total MMAE procedures done (Supplementary Table 2). Relative to the standalone MMAE procedures, the failure rate drops to 0.77% (only 4 standalone MMAE procedures failed out of 516 done). Failure was defined as the need for a rescue surgery or the expansion of the hematoma despite the MMAE procedure. As demonstrated in Supplementary Table 3, 17 studies indicated MMAE as a prophylactic measure for recurrence of symptomatic cSDHs. Out of 859 patients included in those studies, 321 (37.36%) were indicated to MMAE prophylactically. The decision to consider MMAE for recurrence depended on the definition of the risk at which a patient was considered prone for recurrence. The reasons varied according to each article and are summarized in the Supplementary Table 4. Recurrence risk of cSDHs after surgical intervention was assessed in each study differently. Risk factors for recurrence are enlisted per study in the Supplementary Table 5. Of note, usage of OACs and antiplatelet medication was the most common risk factor. Hashimoto, et al., Wang, et al., and Okuma, et al. have cited history of trauma and old age. Alcoholism, liver disease, factor VI deficiency, ESRD, and antiphospholipid syndrome were cited by Link, et al., Shotar, et al., Joyce, et al., Okuma, et al., Ban, et al.
MMAE for recurrence of cSDHs
MMAE was applied for recurrence of cSDHs either with a second surgery done or without any surgical intervention in 15 articles. Out of 556 patients included in those articles, 82 (14.74%) patients had MMAE procedures done without second surgical intervention compared to 34 (6.29%) patients whom have received a second surgical intervention followed by an MMAE procedure. The only article reporting indicating MMAE for both reasons in the same study was Masaki, et al. in 2010.
MMAE for recurrence without surgery: Besides Masaki, et al., of eight articles reporting MMAE procedures done for cSDHs recurrence without a second surgery, two were case reports and six were case series (Supplementary Table 5). Out of 520 patients included in those articles, only 82 had an MMAE procedure for recurrence without a surgery. The remaining patients were indicated for MMAE as either a standalone procedure or prophylactically after the first surgical intervention as by Link, et al., Waqas, et al., Catapano, et al., Joyce, et al., and Nia, et al. The failure rate was 2.23% 12 patients out of 520 patients. The number of failures attributed to MMAE procedures done for recurrence without surgery was not indicated in any article. Hence, the specific failure rate relative to this indication could not be calculated.
MMAE after second surgery for recurrence: Besides Masaki, et al., only 6 articles reported applying MMAE procedures to patients with the aforementioned indication; two were case reports and four are case series (Supplementary Table 6). Except Hashimoto, et al. who reported two MMAE procedures were applied prophylactically; all remaining five articles reported the application of MMAE procedures only after a second surgical intervention after a recurrence. Hence, 34 out of 36 patients had an MMAE procedure after the second surgery. No failures were reported.
Failure of MMAE
MMAE procedures categorized as a failure were either procedures that have resulted in non-resorption of cSDH, increase in the hematoma volume or recurrence of the hematoma after resorption. Out of the 997 MMAE procedures, 22 procedures failed and 19 procedures were complicated (Supplementary Table 7). Hence the failure rate was 4.1%. Compared to Di Cristofori, et al. in March 2022, this failure rate is low (5.5%). This could be attributed to the relative increase in the population size compared to the literature review by Di Cristofori, et al. The inclusion of the retrospective study done by Nia, et al. published in August 2022 increased the population size from 727 to 973 without report of additional failures in MMAE. Reflecting on the population size of the included articles, results of failure rate were similar to the results deducted by Di Cristofori, et al. reflecting on the indication of MMAE application and its corresponding failure rate, a variance has been noted. This could be attributed to the overlap between MMAE indications as for the failure count. Most of the studies did not refer to the failure rate per MMAE indication. Overall, the failure rate diminished with the inclusion of a large study published by Nia, et al. in 2022.
Complications of MMAE
Only five complications were reported in the reviewed articles and it was noticed that all complications happening with patients whose MMAE procedures were indicated as either a standalone or a prophylactic procedure. The complications were a worsening cSDHs, two CVAs, a focal seizure and an episode of aphasia.
Comparison with conventional treatment
In the literature review published by Di Cristofori, et al. in March 2022, the author reported that five studies cited a comparison between patients to whom MMAE was applied versus patients treated conventionally. The implementation of the results reported by Nia, et al. in August 2022 along with the results of the article by Di Cristofori, et al. are depicted in Figure 5. With a similar MMAE group population, Nia, et al. reported much lower rates of failure (0.47% vs. 3.9%). Although a similar result can be deducted concerning the rate of recurrence after conventional technique application, this can be attributed to the large population (4050) studied by Nia, et al. relative to the 727 patients reported by Di Cristofori, et al.
Discussion
Indications for MMA embolization through the literature
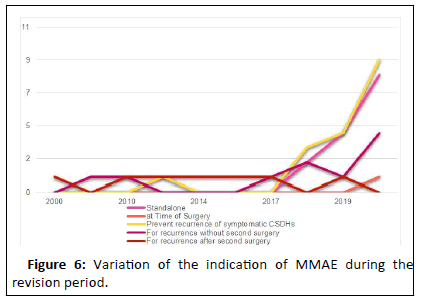
This review of literature unveiled the application of MMAE as for the following indications: A standalone procedure, an adjuvant treatment at time of first surgery, for prevention of recurrence after first surgical evacuation, as a treatment after recurrence after first surgical evacuation without second surgery and as a treatment after recurrence after second surgical evacuation. Keen attention ought to discriminate the first indication versus the other four indications. MMAE as a standalone procedure manages cSDHs by vascular compromise. Hence, further progression of cSDHs by standalone MMAE is valid [21]. The other four scenarios target the mass effect imparted by the hematoma volume by surgical evacuation and target the vascular supply of the dural cell layer by MMAE, yet each at a different stage regarding the surgical intervention and number of recurrences. Therefore, this justifies the higher rate of standalone MMAE procedures failure compared to the other four modalities reported by Di Cristofori, et al. (3.9% versus 8.9%). Meanwhile in this review, the inclusion of the study by Nia, et al. shifted the results in favor of standalone MMAE (3.87% vs. 6.25% and 10.25%). However, Nia, et al. did not feature specific values regarding standalone MMAE procedures done for asymptomatic and symptomatic cSDHs patients. Application of MMAE to patients with asymptomatic cSDHs imparts bias to the results. Similarly, attribution of higher success rate of the combined indications to the application of MMAE shadows 13 of 17 the obvious fact that some patients could have been cured by mere surgical evacuation. The combination with MMAE does not mandate additive benefit. Comparison with a control group for each of the four combined indications is key. Figure 4 shows the variation of the indication of MMAE through the period of this literature review. It is evident that MMAE has been indicated recently as a primary option for therapy of cSDH as either a standalone procedure or adjuvant to primary surgical evacuation in symptomatic patient. However, the eligibility of patients to MMAE remains not clearly defined as shown in Figure 6. Some exclusion criteria reported by authors i.e. patients with midline shift are excluded by Ban, et al., are conversely considered as inclusion criteria by other authors for standalone MMAE i.e. Catapano, et al.
Report of MMAE applications and results
The number of patients to whom MMAE was applied in the reviewed literature conspicuous. Some articles reported the number of the treated patients; others report the number of cSDHs yet not the number of patients. Bilateral cSDHs are reported as two cases and treatment of either have been reported by different modalities for different indications in the same individual. Limited is the report of segregation of patients according to whether the cSDH is unilateral or bilateral. Risk factors of progression and recurrence, results of MMAE application, long-term outcomes, side effects, mortality and morbidity need to be assessed for each of the two patient categories. This is not presented in the literature. Hence, results cannot be generalized. Heterogeneity of the data adds to the lack of parameters related to the number of patients within each category, number of patient considered for each indication, the time between the surgical intervention the MMAE procedure and the type of MMAE material and technique specifications. Moreover, categorization of patients according to morbid conditions, OACs administration and risk factor variance is missing in the articles, as comparison among those groups is not derived.
Future considerations
Despite the aforementioned bias, discrimination between standalone MMAE and MMAE associated to surgical evacuation, regardless of the situation of either procedures, is paramount. Future application of MMAE associated to surgical evacuation is anticipated to provide better results versus standalone MMAE. This was noted previously in the results section. The mere fact that residual hematoma remains in the case of standalone MMAE constrains the efficacy of the latter. This increases its failure rate as patients could fail to resorb the residual volume and rebleeding could be a complication especially in patients with OACs and anti-platelets intake. The necessity to surgically evacuate hematomas and consider MMAE later after surgical intervention appears as a better approach. Future consideration of studies addressing the adequate timeframe is highly recommended. This approach is anticipated to reduce the hospitalization period, the costs of second surgical interventions, promote a better prognosis with less morbidity and mortality especially in the elderly population. Therefore, clinical trials inclusive of standalone MMAE applied to symptomatic yet poor surgical candidates and MMAE procedure applied at different timeframes to surgical candidates after surgical intervention versus conventional control groups are indispensable. The studies published by Nia, et al. and by Ng, et al. are representative of the required studies.
Conclusion
CSDH is a disease of the elderly who struggle with the eventual result of aging: Comorbidities and chronic diseases. Treatment of this bleeding condition within the skull has been conventionally surgical. However, recurrence of this condition and the risks implemented in surgical evacuation of a cSDH renders less invasive procedures more appealing. Hence, the attention drives us towards MMAE. This literature review aimed to provide a walkthrough of the available data about MMAE application for the treatment of cSDH. Anticipation of future applications of MMAE, the indications and the foreseeable outcomes were speculated in light of the reported findings by articles over the last 22 years. As a result, application for the prevention of recurrence and as a primary modality of care has been the indications of choice. However, heterogeneity of the data limited this work and hindered the distinction between indication specific MMAE failure rates. Safety of MMAE as a procedure is regarded as a general theme through the literature and can be considered for future application. We look forward to clinical trials with comparative themes to provide better population specific deductions about this new technique.
Acknowledgment
“No one who achieves success does so acknowledging the help of others. The wise and the confident acknowledge this help with gratitude”, said Alfred North Whitehead.
And I say to all, whom have been of help for the fulfillment of this review.
To Dr. Mohanna for his unconditional assessment during the formulation of the thesis topic and refinement of the aims of this literature review.
To Dr. Shkeir for his guidance and time during the gathering of information and review of imaging at the ZHUMC radiology department. Had this project been without Dr. SHKEIR’s assessment and impressions, the work would have been more laborious and time consuming.
To the jury members Dr. Chehade and Dr. Saad for their keen evaluation, refinements, recommendations and time during the presentation phase and to my family members who have been patient and supportive.
I have immense gratitude.
References
- Tang J, Ai J, Macdonald RL (2011) Developing a model of chronic subdural hematoma. Acta Neurochir Supp 111:25-29
[Crossref] [Google Scholar] [PubMed]
- Yadav Y, Parihar V, Namdev H, Bajaj J (2016) Chronic subdural hematoma. Asian J Neurosurg 11:330-342
[Crossref] [Google Scholar] [PubMed]
- Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, et al. (2017) Traumatic brain injury: Current treatment strategies and future endeavors. Cell Transplant 26:1118-1130
[Crossref] [Google Scholar] [PubMed]
- Ahn JH, Jun HS, Kim JH, Oh JK, Song JH, et al. (2016) Analysis of risk factor for the development of chronic subdural hematoma in patients with traumatic subdural hygroma. J Korean Neurosurg Soc 59:622-627
[Crossref] [Google Scholar] [PubMed]
- Nentwig MJ, Whitaker CM, Yang SY (2019) Spinal subdural hygroma as a post-operative complication in revision spine fusion: A case report. J Surg Case Rep 305
[Crossref] [Google Scholar] [PubMed]
- Rovlias A, Theodoropoulos S, Papoutsakis D (2015) Chronic subdural hematoma: Surgical management and outcome in 986 cases: A classification and regression tree approach. Surg Neurol Int 6
[Crossref] [Google Scholar] [PubMed]
- Lee IH, Choi JI (2022) A retrospective study from a single center of 208 patients with unilateral chronic subdural hematoma to compare outcomes following burr hole craniotomy and hematoma drainage within 48 hours and between 48 hours and 5 days. Int Med Sci Monit 936774-936781.
[Crossref] [Google Scholar] [PubMed]
- Moshayedi P, Liebeskind DS (2020) Middle meningeal artery embolization in chronic subdural hematoma: Implications of pathophysiology in trial design. Front Neurol 11:923
[Crossref] [Google Scholar] [PubMed]
- Bergers G, Song S (2005) The role of pericytes in blood vessel formation and maintenance. Neuro Oncol 7:452-464
[Crossref] [Google Scholar] [PubMed]
- Degen KE, Gourdie RG (2012) Embryonic wound healing: A primer for engineering novel therapies for tissue repair. Birth Defects Research Part C: Embryo Today: Reviews 96:258-270
[Crossref] [Google Scholar] [PubMed]
- Li S, Nguyen IP, Urbanczyk K (2020) Common infectious diseases of the central nervous system clinical features and imaging characteristics. Quant Imaging Med Surg 10:2227
[Crossref] [Google Scholar] [PubMed]
- Tripathy SR, Swarnakar PK, Mishra S, Mishra SS, Dhir MK, et al. (2016) A review of Sub-Acute Subdural Hematoma (SASDH) with our institutional experience and its management by Double Barrel Technique (DBT): A novel technique. Surg Neurol Int 767
[Crossref] [Google Scholar] [PubMed]
- Stanisic M, Hald J, Rasmussen IA, Pripp AH, Ivanovic J, et al. (2013) Volume and densities of chronic subdural haematoma obtained from CT imaging as predictors of postoperative recurrence: A prospective study of 107 operated patients. Acta Neurochir (Wien). 155:323-333
[Crossref] [Google Scholar] [PubMed]
- Zhu F, Wang H, Li W, Han S, Yuan J, et al. (2022) Factors correlated with the postoperative recurrence of chronic subdural hematoma: An umbrella study of systematic reviews and meta-analyses. Clin Med 43:101234
[Crossref] [Google Scholar] [PubMed]
- Kwon SM, Lee MH, Seo Y, Kim YI, Oh HJ, et al. (2022) A radiological assessment of chronic subdural hematomas. Korean J Neurotrauma 18:12
[Crossref] [Google Scholar] [PubMed]
- Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KL, Hutchinson PJ (2017) Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation 14:1-3
[Crossref] [Google Scholar] [PubMed]
- Case ME (2008) Inflicted traumatic brain injury in infants and young children. Brain pathol 18:571-582
[Crossref] [Google Scholar] [PubMed]
- Zolal A, Sobottka SB, Podlesek D, Linn J, Rieger B, et al. (2016) Comparison of probabilistic and deterministic fiber tracking of cranial nerves. J Neurosurg. 127:613-621
[Crossref] [Google Scholar] [PubMed]
- Oh JW, Kim SH, Whang K (2017) Traumatic cerebrospinal fluid leak: Diagnosis and management. Korean J Neurotrauma 13:63
[Crossref] [Google Scholar] [PubMed]
- Lee KS (2015) History of chronic subdural hematoma. Korean J Neurotrauma 11:27
- Stubbs DJ, Vivian ME, Davies BM, Ercole A, Burnstein R, et al. (2021) Incidence of chronic subdural haematoma: A single center exploration of the effects of an ageing population with a review of the literature. Acta Neurochirurgica 163:2629-2637.
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences