The Efficacy of Clipping over Endovascular Coiling In Reducing Shunt Dependent Hydrocephalus after Rupture of Intracranial Aneurysms, an Institutional Experience
Vikas Chandra Jha*, Mohammad Shahnawaz Alam, Vivek Saran Sinha and Rahul Jain
Department of Neurosurgery, All India Institute of Medical Sciences, Bihar, India
- *Corresponding Author:
- Vikas Chandra Jha
Department of Neurosurgery,
All India Institute of Medical Sciences,
Bihar,
India,
Tel: 917417623505;
E-mail: drshahnawaza@aiimspatna.org
Received date: October 15, 2022, Manuscript No. IPJNCS-22-14778; Editor assigned date: October 18, 2022, PreQC No. IPJNCS-22-14778 (PQ); Reviewed date: November 01, 2022, QC No. IPJNCS-22-14778; Revised date: December 27, 2022, Manuscript No. IPJNCS-22-14778 (R); Published date: January 04, 2023, DOI: 10.36648/2471-9633.7.1.001
Citation: Jha VC, Alam MS, Sinha VS, Jain R (2023) The Efficacy of Clipping over Endovascular Coiling In Reducing Shunt Dependent Hydrocephalus after Rupture of Intracranial Aneurysms, an Institutional Experiencee. Neurosurg Vol:7 No:1
Abstract
Objectives: Clipping and Endovascular Therapy (EVT) have a variable benefit in reducing Shunt Dependence Hydrocephalus (SDHC) following an aneurysm bleed. We compared clipping over endovascular treatment in reducing such dependence. Additionally, we tried to assess the effect of lesser-studied modifiable risk factors such as External Ventricular Drainage (EVD), Ommaya reservoir placement, CSF protein, cisternostomy and vasospasm.
Materials and methods: We retrospectively analyzed 67 out of 300 patients treated between July 2018 and December 2021 at our center for ruptured aneurysms who developed hydrocephalus following an aneurysmal bleed. We divided the patients into two groups, one undergoing clipping other EVT.
Results: Of 67 patients, 33 were treated by clipping and 34 by coiling. Compared to clipping, coiling could not significantly reduce shunt dependence (p=0.66). On stepwise logistic regression analysis of risk factors for SDHC, the timing of EVD (p=0.05) and CSF protein on day 7 (p=0.01) was a significant risk factor. Ommaya reservoir placement following EVD was found to reduce shunt dependence (p=0.0001), together with patients responding to spasmolysis following vasospasm (p=0.001) and fenestration of lamina terminalis (p=0.017), while clipping.
Conclusion: We did not find any significant advantage of clipping over endovascular therapy in reducing shuntdependent hydrocephalus following aneurysmal rupture. The duration taken to put EVD after the onset of hydrocephalus and CSF protein on the seventh day, reflecting persistent infection or higher blood degradation, can also influence shunt dependence. Ommaya reservoir placement can reduce shunt dependence in patients who underwent EVD earlier and can substitute it, requiring further prospective study to comment certainly the use of Ommaya reservoir as a substitute for EVD for external CSF drainage in hydrocephalus induced by aneurysmal bleed.
Keywords
Hydrocephalus; Aneurysm rupture; Clipping versus coiling; Shunt dependence
Abbreviations:
CT: Computed Tomography; DACA: Distal Anterior Cerebral Artery; DSA: Digital Subtraction Angiography; EVT: Endovascular Therapy; GOS: Glasgow Outcome Score; ICU: Intensive Care Unit; mRS: modified Rankin Score; SAH: Subarachnoid Hemorrhage; SDHC: Shunt Dependent Hydrocephalus
Introduction
Shunt Dependent Hydrocephalus (SDHC) develops in 6%-60% of cases following Subarachnoid Hemorrhage (SAH) [1]. Few studies have shown the advantage of clipping over coiling [2]. However, a recent meta-analysis does not suggest any significant difference in reducing the incidence of SDHC in the long term [3]. This hydrocephalus often responds to cisternostomy while performing aneurysm clipping and by putting external ventricular drainage containing a long tube system with a drainage bag or intermittent drainage through the Ommaya reservoir perioperatively in patients undergoing clipping or Endovascular Therapy (EVT). In 40-50% of such patients removing EVD leads to ventriculomegaly and clinical deterioration, due to which a ventriculoperitoneal shunt needs to be placed subsequently for persisting hydrocephalus. Continuous drainage through EVD has the inherent risk of infection (0%-45%), tube blockage and intracranial hemorrhage [4]. There is growing evidence emphasizing intermittent and slow drainage of CSF leading to lesser chances of EVD related complications by replacement of EVD by Ommaya reservoir, thus reducing chances of infection in some studies. Few studies have reported reduced shunt dependency in hydrocephalus following SAH when CSF is drained intermittently and gradually compared to continuous drainage and fast weaning [5]. Few studies suggested a reduction in complications associated with intermittent EVD drainage as they decrease the requirement of ventriculoperitoneal shunts, which can either be achieved by intermittently closing the EVD tube or placing the Ommaya reservoir draining intermittently [6]. Risk factors such as age, Infarct on CT head, higher hunt and hess grade and intraventricular hemorrhage with acute dilatation have been documented commonly as risk factors associated with the development of shunt dependent hydrocephalus following aneurysmal bleed and these risk factors cannot be modified [7]. Some studies compared the scoring system, including these factors but comparing these scoring systems by external validation suggested its insufficiency in predicting shunt dependency [8]. Very few studies have included the effect of cisternostomies, EVD, Ommaya reservoir placement and spasmolysis, which we regularly perform as an adjunctive procedure to clipping and EVT. These can affect shunt dependency and are directly responsible for developing shunt-dependent hydrocephalus. Their effect can be modified to reduce shunt dependency in such circumstances in patients either being treated by clipping or Endovascular Therapy (EVT) [9-11]. Given these facts, we tried to compare the efficacy of microsurgical clipping over Endovascular Treatment (EVT) in preventing shunt dependent hydrocephalus after subarachnoid hemorrhage in patients with a ruptured aneurysm. The secondary objective was to assess the effect of additional procedures such as EVD insertion, Ommaya reservoir placement, cisternostomy, age, gender, meningitis, hunt and hess grade, Fischer grade, intraventricular bleed, Infarct and vasospasm causing shunt dependent hydrocephalus, which could have affected the outcome of the primary procedure (clip versus endovascular therapy).
Materials and Methods
Following the approval from the institutional ethical committee, All India Institute of medical sciences, Patna, with reference number AIIMS/Pat/IEC/2021/278, the study was conducted between July 2018 and December 2021 in the neurosurgery department at our institute. In this duration, 300 cases with aneurysmal Subarachnoid Hemorrhage (aSAH) with intracranial bleed at other locations also were treated. We included 67 patients in the study who developed acute hydrocephalus as observed on repeat NCCT head in the ward after admission or follow-up following treatment which was not present at the NCCT head immediately after ictus.
Age, gender, initial glasgow coma scale, comorbidities, hunt and hess grade, Fischer score on computed tomography, EVD placement duration, complications, Ommaya reservoir placement, CSF drainage (continuous/intermittent), CSF protein measurement, glasgow outcome score at follow up, modified Rankin score at follow up were noted and analysed.
Radiological findings on the NCCT head as Fischer CT grade, Evan's index, periventricular-lucency and other investigation findings such as preoperative and postoperative DSA and MRI brain were noted. DSA and spasmolysis by microcatheter injection of intraarterial nimodipine were performed if we observed vasospasm on CT angiography. Patients with deteriorating neurological conditions following the procedure and not showing improvement underwent CT angiography. The other laboratory data were preoperative and postoperative routine blood investigations and CSF routine microscopy sent at regular intervals.
The accessory surgical procedure performed besides clipping and endovascular therapy
EVD or ommaya reservoir insertion: It was indicated in all patients with hydrocephalus on the NCCT head and patients with altered sensorium (GCS<15). It was placed preoperatively, 2.5 cm lateral to the midline and 1 cm in front of the coronal suture, by placing a 1.5 cm straight incision on the coronal suture. The Ommaya reservoir was indicated in a patient with persistent infection as observed through CSF drainage fluid through EVD and frequent blockage of EVD. Its tapping was done with no 16 scalp vein set with its tubings and kept under sterile transparent dressings.
EVD was opened intermittently to drain 50 ml-100 ml depending upon ICP measurement of more than 20 cm or lower. It was kept on continuous drainage after treating the aneurysms by endovascular coiling or microsurgical clipping. Once the patient started improving postoperatively, the patients were gradually weaned off from EVD by intermittently opening the EVD. Meanwhile, we strictly monitored any drop in GCS and rise in blood pressure to look for features of raised ICP. A trial of weaning from EVD was given every 24 hrs in patients with Hunt and Hess grades 1-2 and 48 hrs in grades 3-4. During these repeated trials, a few patients had hardware complications such as frequent blockage and infections, for which we replaced it with the Ommaya reservoir. Placement of the Ommaya reservoir act as a conduit for intermittent CSF drainage and antibiotics instillation, which helped us decrease intracranial infection and gradual and intermittent EVD drainage.
After the Ommaya reservoir placement, we used to assess the requirement of CSF tapping through it depending upon the GCS status of the patient chart at regular intervals in the critical care monitoring sheet. In patients requiring more frequent drainage of CSF through scalp vein set (>3-4 times/day) regularly for 4-5 days, we used to wait for the CSF sample to become sterile and replace it with a ventriculoperitoneal shunt. We reduced the ommaya reservoir tapping once a day to 24 hourly and 72 hourly and subsequently stopped after 3-4 trials of 72 hrs tap trials and stable GCS. It was difficult to consistently measure opening CSF pressure or ICP using various monitoring equipment and we could not do this in all patients included in the study. We waited for the patient to pass the period susceptible to vasospasm or for the patient to have no features suggestive of vasospasm angiographically and clinically. The length of stay in the ICU and hospital, the patient's Glasgow outcome score and the modified Rankin Scale (mRS) score from follow-up records were noted.
Statistical analysis
All statistical analyses were done using Statistical Package for the Social Sciences (SPSS version 20.0; IBM Corp., Armonk, NY, USA). Multiple logistic regression analyses were done to identify the risk factors for developing shunt dependent hydrocephalus following aSAH. Continuous variables using a t-test as appropriate were compared and categorical variables were expressed as numbers (percentages) and compared using the chi-square test or Fisher's exact test. Factors with a p-value of less than 0.05 in the univariate analysis were entered into the stepwise logistic regression analysis. A two-tailed p-value of less than 0.05 was considered statistically significant.
Results
Three hundred patients with ruptured aneurysms with SAH were treated, of which 67 (26.8%) developed hydrocephalus during the course. 33 patients of these 67 patients were treated by microsurgical clipping and 34 by endovascular therapy. The average age of the clipping group of patients was 47.66 ± 8.6 years and 54.17 ± 10.30 years in the Endovascular Therapy (EVT) group (p=0.007). Male and female ratios of patients treated by clipping were 17:16 (1.06) and in the EVT group was 0.7 with no significant difference (p=0.39). Twenty-two patients in the clipping group had aneurysms located in the anterior and 11 in the posterior circulation. Ten patients in the coiling group had aneurysms in the anterior and 24 in the posterior circulation, with posterior circulation aneurysm cases more prone to develop hydrocephalus (p=0.002). Preoperative hunt and hess grade was 3-4 in 33 patients in the clipping group and 34 patients in the coiling group (p=0.44) and an mRS score of more than 2 was present in 24 patients in the clipping group and 22 patients with coiling group(p=0.54). On the NCCT head, 19 patients had intra-parenchymal bleed compared to 10 patients in the coiling group, with 22 patients in clipping and 26 in the coiling group having intraventricular bleed (p=0.04). Perioperative Infarct was present in 24 patients in the clipping and coiling group (p=0.24) and these patients had vasospasm on DSA, which we detected irst a ter CT angiography following neurological deterioration. Following intraarterial microcatheter injection of Nimodipine, ten patients in clipping and 14 in the coiling group responded (Table 1).
| Variables | Clipping (n=33) | Coiling (n=34) | P-value |
|---|---|---|---|
| Age (years) | 47.66 ± 8.6 | 54.17 ± 10.30 | 0.007 |
| Gender | 17:16 | 14:20 | 0.39 |
| Location of the aneurysm | |||
| Anterior circulation | 22 | 10 | |
| Posterior circulation | 11 | 24 | 0.002 |
| Hunt and hess grade | |||
| 3-5 | 33 | 34 | 0.44 |
| mRS score (Pre-op) | |||
| 1-2 | 9 | 12 | |
| 3-4 | 24 | 22 | 0.54 |
| Fischer grade | |||
| 1 | 4 | 2 | 0.058 |
| 2 | 10 | 8 | |
| 3 | 16 | 20 | |
| 4 | 3 | 4 | |
| CT finding SAH | 33 | 34 | |
| Intraparenchymal bleed | 19 | 10 | |
| Intraventricular bleed | 10 | 25 | 0.04 |
| Post procedure infarct with vasospasm | |||
| Present | 24 | 24 | 0.024 |
| Absent | 9 | 10 | |
| Spasmolysis | |||
| Responded | 10 | 14 | 0.68 |
| EVD performed (days) | |||
| Average duration to perform EVD | 8.39 ± 5.42 | 6.43 ± 5.26 | 0.04 |
| Average duration on EVD | 10.49 ± 6.41 | 13.64 ± 8.29 | 0.18 |
| Average duration on Ommaya reservoir | 11.69 ± 6.36 | 12.34 ± 9.48 | 0.17 |
| Stroke | 2 | 3 | |
| CAD | 2 | 2 | |
| Hypertension | 14 | 19 | 0.23 |
| Diabetes | 12 | 14 | 0.21 |
| CKD | 1 | 1 | |
Table 1: Risk factors associated with clipping versus coiling for hydrocephalus following SAH.
Of these 24 who responded to spasmolysis, 20 did not require ventriculoperitoneal shunt for hydrocephalus following aneurysmal SAH. Still, the remaining 24 did not respond to spasmolysis; only five were shunt independent on follow-up (Table 2). 12 among the 33 patients who underwent clipping did not require a shunt on follow-up, but among coiling, ten patients out of 34 on follow-up (p=0.66) (Table 3). We performed lamina terminalis fenestration in 13 patients who underwent clipping; eight did not require a shunt on follow-up. There were 20 patients in whom cisternostomies were done, but lamina terminalis fenestration was not done and only four did not require a shunt (p=0.017) (Table 4).
| Shunt dependence | Response to spasmolysis | n (%) | |
|---|---|---|---|
| Present | Absent | ||
| Present | 4 | 19 | 23 (47.9%) |
| Absent | 20 | 5 | 25 (52.1%) |
| 24 (50.0%) | 24 (50.0%) | 48 | |
| Chi-squared | 18.391 | ||
| df | 1 | ||
| Significance level | P<0.0001 | ||
| Contingency coefficient | 0.526 | ||
Table 2: Spasmolysis and shunt dependence in hydrocephalus following aneurysm rupture.
| Shunt dependent | Procedure | n (%) | P-value | |
|---|---|---|---|---|
| Clipping | Coiling | |||
| Present | 23 | 22 | 45 (67.2%) | 0.66 |
| Absent | 12 | 10 | 22 (32.8%) | |
| 33 (49.3%) | 34 (50.7%) | 67 | ||
| Chi-squared | 0.186 | |||
| df | 1 | |||
| Significance level | P=0.6660 | |||
| Contingency coefficient | 0.053 | |||
Table 3: Clipping versus Endovascular Therapy (EVT) and shunt dependence hydrocephalus following an aneurysmal bleed.
| Shunt dependence | Cisternostomy | n (%) | |
|---|---|---|---|
| With lamina terminalis fenestration | Without lamina terminalis fenestration | ||
| Present | 5 | 16 | 21 (63.6%) |
| Absent | 8 | 4 | 12 (36.4%) |
| 13 (39.4%) | 20 (60.6%) | 33 | |
| Chi-squared | 5.697 | ||
| df | 1 | ||
| Significance level | P=0.0170 | ||
| Contingency coefficient | 0.384 | ||
Table 4: Cisternostomy and shunt dependence in hydrocephalus following aneurysm rupture.
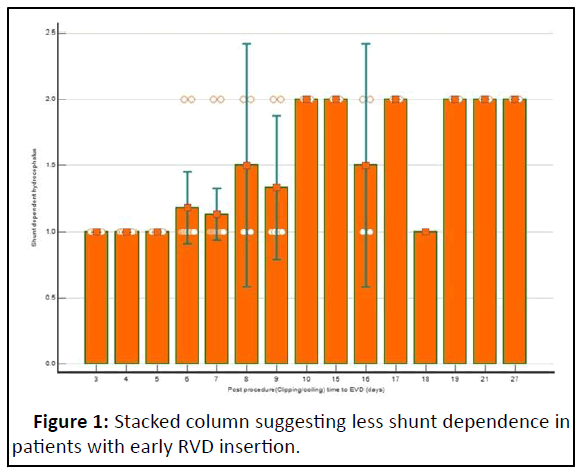
The average duration to perform EVD was 8.39 ± 5.4 and in the coiling group, 6.43 ± 5.26 days (p=0.04) with a minimum of 3 days and a maximum of 17 days. Patients in whom EVD was performed early were less shunt dependent compared to patients in whom EVD was placed late (Figure 1).
EVD was required in 7 out of 9 patients who underwent cisternostomy with lamina terminalis fenestration and 14 out of 24 who underwent cisternostomy without lamina terminalis fenestration. These were patients in whom clipping was performed. In patients who underwent Endovascular Therapy (EVT), EVD was required in 33 out of 34 patients and one responded to conservative treatment (p=0.018). Altogether EVD was put in 54 patients. 19 out of 54 whom EVD were placed for CSF drainage independent of the Ventriculoperitoneal shunt (VP shunt) on follow-up. Still, three patients out of 13 in whom EVD was not placed were independent of VP shunt (p=0.40) on follow-up. EVD placement does not appear to affect shunt dependency (p=0.40) significantly (Table 5).
| Shunt dependent | EVD performed | n (%) | |
|---|---|---|---|
| Present | Absent | ||
| Present | 35 | 10 | 45 (67.2%) |
| Absent | 19 | 3 | 22 (32.8%) |
| 54 (80.6%) | 13 (80.6%) | 67 | |
| Chi-squared | 0.686 | ||
| df | 1 | ||
| Significance level | P=0.4075 | ||
| Contingency coefficient | 0.101 | ||
Table 5: EVD placement and shunt dependency in patients with hydrocephalus following an aneurysmal bleed.
Ommaya reservoir placement was done in 25 patients who required EVD initially (n=54). It was required in 4 out of 9 patients in whom EVD was not done in a later stage due to meningitis picture in CSF (p=0.13). We observed that ten patients out of 29 in whom the Ommaya reservoir was placed were shunt-dependent. Still, 22 patients out of 25 in whom the Ommaya reservoir was not placed were shunt-dependent (p=0.001) (Table 6).
| Shunt dependent | Ommaya reservoir placement | n (%) | |
|---|---|---|---|
| Present | Absent | ||
| Present | 10 | 22 | 32 (59.25%) |
| Absent | 19 | 3 | 22 (40.75%) |
| 29 (43.3%) | 38 (56.7%) | 67 | |
| Chi-squared | 24.394 | ||
| df | 1 | ||
| Significance level | P<0.0001 | ||
| Contingency coefficient | 0.517 | ||
Table 6: Ommaya reservoir placement and shunt dependency in patients with hydrocephalus following an aneurysmal bleed.
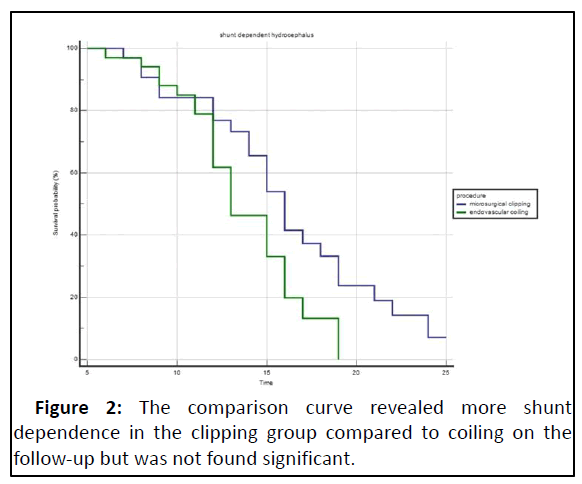
Risk factors that were found significant on univariate analysis as age, hunt and hess grade, Fischer grade on CT, periprocedural Infarct on CT, acute hydrocephalus, CSF protein measurements done for the first seven days following EVD and Ommaya reservoir tap, procedure (clip vs. coil), duration to perform EVD, additional procedure as EVD and Ommaya reservoir placement were put forth for stepwise logistic regression analysis. We found the time taken (in days) to perform EVD as significantly contributing (p=0.05), together with CSF protein on day 7 (p=0.01) (Table 7). Patients who were shunt dependent were more in Clipping than in the coiling group, but this difference was insignificant (P=0.6). mRS score at six months of follow-up and glasgow outcome score was almost similar in both the groups of patients (p=0.21 and p=0.26). The ICU and hospital stay length was significantly better in coiling than in the clipping group (p=0.0001) (Table 8 and Figure 2).
| Variable | Coefficient | Std. Error | Odds ratio | 95% CI | P-value |
|---|---|---|---|---|---|
| Cisternostomy | -3.27836 | 2.15803 | 0.0377 | 0.0005-2.5893 | 0.12 |
| Duration to perform EVD (days) | 0.98201 | 0.50606 | 2.6698 | 0.9902-7.1987 | 0.05 |
| CSF protein day 7 | 0.013029 | 0.00834 | 1.0131 | 0.9967-1.0298 | 0.01 |
| Constant | -4.52028 | 3.33772 | 0.17 |
Table 7: Stepwise multiple logistic regression analysis for risk factors responsible for shunt dependent hydrocephalus following an aneurysmal bleed.
| mRS scale postoperative | Microsurgical clipping | Endovascular coiling | n (%) | P- value |
|---|---|---|---|---|
| No symptoms | 0 | 0 | ||
| Able to carry all work with symptoms | 1 | 6 | 9 (11.9%) | 0.205 |
| Unable to carry all previous work | 2 | 4 | 6 (8.95 %) | |
| Able to walk unassisted | 14 | 14 | 28 (41.8%) | |
| Unable to walk unassisted | 14 | 10 | 24 (35.8%) | |
| Glasgow outcome score | ||||
| (Death) | 2 | 2 | 4 (5.9%) | 0.288 |
| (Neurovegetative state) | 1 | 0 | 3 (4.47%) | |
| (Severe disability pt dependent for daily work) | 17 | 10 | 27 (40.3%) | |
| (Moderate disability (pt independent in daily life) | 12 | 15 | 27 (40.3%) | |
| (Resumption of normal life with minor neurological deficits | 2 | 7 | 9 (13.43%) | |
| Shunt dependent | 21 | 24 | 0.66 | |
| Shunt independent | 12 | 10 | ||
| Length of stay in ICU (days) | 23.60 ± 6.29 | 9.27 ± 2.6 | 0.001 | |
| Length of stay in hospital (days) | 33.6 ± 7.9 | 18.72 ± 2.19 | 0.001 | |
| Mortality | 2 | 2 | ||
Table 8: Comparision of the outcome at follow-up in patients who have undergone clipping versus EVT and have hydrocephalus following ruptured aneurysm bleed following.
Discussion
Different studies have reported the importance of surgical clipping over EVT in reducing the SDHC due to addressing the blood clot, which produces obstruction and inflammatory changes in the CSF draining pathway [12]. Hydrocephalus following SAH has been reported to be communicating type due to inadequate functioning of arachnoid granulation and inflammatory fibrosis in cisterns. Hydrocephalus following ruptured aneurysmal bleed may develop with variable latency depending upon inflammatory fibrosis in the CSF outlet pathway.
Komotar, et al., in a systemic review, reported no difference in Shunt Dependent Hydrocephalus (SDH) following SAH in the surgical clipping group with and without lamina terminalis fenestration [13]. In our study, patients with lamina terminalis fenestration which helped in early clot removal from the ventricle with subsequent EVD for CSF drainage were less shunt dependent than those with no lamina terminalis fenestration. It may be due to the combined effect of reducing blood clot burden and continuous CSF drainage leading to a decrease in blood degradation product-induced inflammatory response in the CSF drainage pathway, which leads to reduced SDHC.
The average time of EVD replacement in the study by Varela, et al. was 17 days and in Erixon, et al., 45 days [14]. The average time in the present study was lower than that of the abovereported studies. In the meta-analysis by Xie, et al., the minimal duration noted was 17 days and the maximum duration was 45 days. In our study decision of early EVD was taken because of deteriorating neurological status and increasing ventricular dilatation on repeat CT head. These features may have happened due to the higher Fischer grade on the CT head in patients in the present study leading to CSF outlet obstruction earlier. We observed in our patients that the earlier the decision to perform the EVD was taken, the lesser the chance of VP shunt. It may be due to the earlier removal of blood degradation products responsible for inducing inflammation and fibrosis. It was supplemented by the fact that higher CSF protein on the fifth postoperative day of EVD placement was the major predictor of shunt dependency as it is the marker of surrounding inflammatory response and burden of a blood degradation product. In the study by Lenski, et al. and Lewis, et al., CSF protein on the fifth day of EVD insertion strongly predicted SDHC. The importance of inserting EVD early after noticing hydrocephalus following SAH was also stressed in other studies [15].
Insertion of EVD for external CSF drainage after acute hydrocephalus following SAH has inherent complications such as mechanical obstruction and an increased risk of intracranial infection if prolonged. Many studies have found it an additional risk factor for shunt-dependent hydrocephalus. Studies have reported an increased incidence of meningitis following EVD tube placement and frequent opening, closing and keeping it longer. Studies suggest an increased risk of developing meningitis in intensive care units where patients with tracheostomy, other fluid draining systems and more crowded as in our setup, have more meningitis with EVD tube [16,17]. Similar was the observation in the present study, where meningitis with EVD insertion was higher than in the reported studies leading to more chances of shunt dependency. We tried to minimize this by replacing the EVD tube system with an Ommaya reservoir which helps to reduce creeping infection and frequent manipulation of the EVD tube. Intermittent drainage through the ommaya tube and the instillation of antibiotics helped us reduce meningitis and shunt dependency in our patients. Intermittent EVD clamping and CSF drain through the Ommaya reservoir may reduce shunt dependence. However, it requires careful patient monitoring and persistence, as observed in the present study, where resources are scarce and ICU is overcrowded a common scenario in developing countries.
In contrast to Klopfenstein, et al. which focused on gradual weaning with a single clamp trial and reported more patients needing VP shunts at the end, Ascanio, et al. had used multiple trials of EVD clamping before inserting a shunt, which led to a decreased number of cases that were shunt dependent. The contradictory findings in these studies may have been observed due to EVD-related complications, which were higher in the latter study [18,19]. Klopfenstein, et al. reported a single clamping trial in their study because of which they reported 63% shunt dependent cases in follow up but in the study it was multiple trials of clamping with intermittent CSF drainage, which resulted in 17% shunt dependent cases [20,21]. We can substitute this practice of intermittent clamping of EVD tube by Ommaya reservoir placement as in the present study. The reason for less VP shunt dependence with the Ommaya reservoir in the present study may also be due to more attempts with intermittent drainage and lesser complications such as blockage and infection than EVD, which permitted more time to help the patient the establishment of natural CSF drainage pathway. Arts and Singh, made similar observations in their studies [22,23].
In our study, patients detected to have clinical vasospasm (neurological deterioration with vascular spasm noted on CT angiography and DSA subsequently suspecting it) had more requirements of EVD. Most patients who responded to spasmolysis were stabilized following EVD insertion and were not dependent on follow-up. However, those who did not respond to spasmolysis developed shunt dependency later. Different studies have suggested high CSF protein due to inflammatory conditions prevailing during this condition [24]. In the study, clinical vasospasm was treated by triple H therapy, but shunt dependency in such circumstances was high. Still, we performed direct arterial vasodilation by microcatheter to detect vasospasm on DSA [25,26]. Direct spasmolysis probably works better in such cases and helps in the early reduction of vasospasm, inflammation and CSF protein, which leads to reduced duration to continue EVD and shunt requirements similar to our study.
Rao, et al. reported decreased ICU stay, hospital stay and better Glasgow outcome scores in the group with intermittent CSF drainage and rapid weaning, which is similar to the present study, but differs from the study of Klopfenstein, et al., who have reported decreased ICU and hospital study but the increased rate of shunt dependent cases on follow up.
Conclusion
Microsurgical clipping may not significantly reduce shunt dependence in hydrocephalus following subarachnoid hemorrhage compared to endovascular therapy. Still, minor procedures such as earlier EVD insertion, judicious ommaya reservoir placement and intraarterial microcatheter spasmolysis may reduce shunt dependence in such circumstances. A few studies have reported risk factors such as CSF protein to influence dependence on the shunt, but its role in such a scenario cannot be ignored. Switching to the Ommaya reservoir to replace EVD helps reduce shunt dependence. The earlier this decision is taken, the better it would be as it decreases the incidence of meningitis due to EVD and provides a prolonged window period to decide the lesser requirement of the ventriculoperitoneal shunt, which has lots of shunt-related complications reported in the literature. We need to prospectively validate our results in the large patient group which may improve outcomes in such circumstances.
Declaration
Ethics approval and consent to participate: Written informed consent was taken from patients/relatives at admission to use their data for teaching and research. Approval of the institutional ethical committee, all India institute of medical sciences, Patna, with reference number AIIMS/Pat/IEC/ 2021/278, was taken to conduct this study.
Consent for Publication
Not applicable.
Availability of Data and Material
It will be produced at a reasonable request from the corresponding author.
Competing Interests
There is no conflict of interest among authors.
Funding
Authors declare that they have not received funding for this study.
Authors' Contributions
Conceptualization, clinical work, data collection, data analysis, manuscript drafting and revisions were done by Vikas Chandra Jha. Mohammad Shahnawaz Alam, Vivek Sharan Sinha and Rahul Jain did data collection and analysis. All the authors have read and approved the final version of the manuscript. No conference or scientific meeting has ever included this manuscript in its entirety or as a standalone presentation. This article has not yet been published or is being considered for publication elsewhere.
Acknowledgments
Not applicable.
References
- Koyanagi M, Fukuda H, Saiki M, Tsuji Y, Lo B, et al. (2018) Effect of choice of treatment modality on the incidence of shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg 130:949-955
[Crossref] [Google Scholar] [PubMed]
- Zaidi HA, Montoure A, Elhadi A, McDougall CG, Albuquerque FC, et al. (2015) Long-term functional outcomes and predictors of shunt-dependent hydrocephalus after treatment of ruptured intracranial aneurysms in the BRAT trial: Revisiting the clip vs coil debate. Neurosurgery 76:608-614
[Crossref] [Google Scholar] [PubMed]
- Xie Z, Hu X, Zan X, Lin S, Li H, et al. (2017) Predictors of shunt dependent hydrocephalus after aneurysmal subarachnoid hemorrhage? A systematic review and meta-analysis. World Neurosurg 106:844-860
[Crossref] [Google Scholar] [PubMed]
- Elsharkawy AA, Abdelhameed EA (2020) Efficacy of translamina terminalis ventriculostomy tube in the prevention of chronic hydrocephalus after aneurysmal subarachnoid hemorrhage. Surg Neurol Int 11:283
[Crossref] [Google Scholar] [PubMed]
- Komotar RJ, Hahn DK, Kim GH, Garrett MC, Merkow MB, et al. (2009) Efficacy of lamina terminalis fenestration in reducing shunt-dependent hydrocephalus following aneurysmal subarachnoid hemorrhage: A systematic review. Clinical article. J Neurosurg 111:147-154
[Crossref] [Google Scholar] [PubMed]
- Varelas P, Helms A, Sinson G, Spanaki M, Hacein-Bey L (2006) Clipping or coiling of ruptured cerebral aneurysms and shunt-dependent hydrocephalus. Neurocrit Care 4:223-228
[Crossref] [Google Scholar] [PubMed]
- Erixon HO, Sorteberg A, Sorteberg W, Eide P (2014) Predictors of shunt dependency after aneurysmal subarachnoid hemorrhage: Results of a single-center clinical trial. Acta Neurochir 156:2059-2069
[Crossref] [Google Scholar] [PubMed]
- Vyas D, Booker J, Smith D, Al-Tamimi YZ (2021) External validation of scoring models to predict shunt insertion after aneurysmal subarachnoid hemorrhage. World Neurosurg 146:1255-1261
[Crossref] [Google Scholar] [PubMed]
- Lenski M, Biczok A, Huge V, Forbrig R, Briegel J, et al. (2018) Role of cerebrospinal fluid markers for predicting shunt-dependent hydrocephalus in patients with subarachnoid hemorrhage and external ventricular drain placement. World Neurosurg 121:e535-e542
[Crossref] [Google Scholar] [PubMed]
- Lewis A, Irvine H, Ogilvy C, Kimberly WT (2015) Predictors for delayed ventriculoperitoneal shunt placement after external ventricular drain removal in patients with subarachnoid hemorrhage. Br J Neurosurg 29:219-224
[Crossref] [Google Scholar] [PubMed]
- Yu H, Zhan R, Wen L, Shen J, Fan Z (2014) The relationship between risk factors and prognostic factors in patients with shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. J Craniofac Surg 25:902-906
[Crossref] [Google Scholar] [PubMed]
- de Oliveira JG, Beck J, Setzer M, Gerlach R, Vatter H, et al. (2007) Risk of shunt dependent hydrocephalus after occlusion of ruptured intracranial aneurysms by surgical clipping or endovascular coiling: a single institution series and meta-analysis. Neurosurgery 61:924-934
[Crossref] [Google Scholar] [PubMed]
- Lai L, Morgan MK (2013) Predictors of in hospital shunt dependent hydrocephalus following rupture of cerebral aneurysms. J Clin Neurosci 20:1134-1138
[Crossref] [Google Scholar] [PubMed]
- Hirashima Y, Hamada H, Hayashi N, Kuwayama N, Origasa H, et al. (2003) Independent predictors of late hydrocephalus in patients with aneurysmal subarachnoid hemorrhage-analysis by the multivariate logistic regression model. Cerebrovasc Dis 6:205-210
[Crossref] [Google Scholar] [PubMed]
- Hussein K, Rabino G, Feder O, Eghbaryeh H, Zayyad H, et al. (2019) Risk factors for meningitis in neurosurgical patients with cerebrospinal fluid drains: Prospective observational cohort study. Acta Neurochir (Wien) 161:517-524
[Crossref] [Google Scholar] [PubMed]
- Hagel S, Bruns T, Pletz MW, Engel C, Kalff R, et al. (2014) External ventricular drain infections: Risk factors and outcome. Interdiscip Perspect Infect Dis 2014:708531
[Crossref] [Google Scholar] [PubMed]
- Scheithauer S, Burgel U, Ryang YM, Haase G, Schiefer J, et al. (2009) Prospective surveillance of drain associated meningitis/ventriculitis in neurosurgery and neurological intensive care unit. J Neurol Neurosurg Psychiatry 80:1381-1385
[Crossref] [Google Scholar] [PubMed]
- Ascanio LC, Gupta R, Adeeb N, Moore JM, Griessenauer CJ, et al. (2018) Relationship between external ventricular drain clamp trials and ventriculoperitoneal shunt insertion following nontraumatic subarachnoid hemorrhage: A single center study. J Neurosurg 130:956-962
[Crossref] [Google Scholar] [PubMed]
- Klopfenstein JD, Kim LJ, Feiz-Erfan I, Hott JS, Goslar P, et al. (2004) Comparison of rapid and gradual weaning from external ventricular drainage in patients with aneurysmal subarachnoid hemorrhage: A prospective randomized trial. J Neurosurg 100:225-229
[Crossref] [Google Scholar] [PubMed]
- Rao SS, Chung DY, Wolcott Z, Sheriff F, Khawaja AM, et al. (2019) Intermittent CSF drainage and rapid EVD weaning approach after subarachnoid hemorrhage: Association with fewer VP shunts and shorter length of stay. J Neurosurg 132:1-6
[Crossref] [Google Scholar] [PubMed]
- Olson DM, Zomorodi M, Britz GW, Zomorodi AR, Amato A, et al. (2013) Continuous cerebral spinal fluid drainage associated with complications in patients admitted with subarachnoid hemorrhage. J Neurosurg 119:974-980
[Crossref] [Google Scholar] [PubMed]
- Arts S, Van Lindert EJ, Aquarius R, Bartels RHMA, Boogaarts HD (2021) Complications of external cerebrospinal fluid drainage in aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien) 163:1143-1151
[Crossref] [Google Scholar] [PubMed]
- Singh H, Patir R, Vaishya S, Miglani R, Kaur A (2020) External ventricular drain related complications-whether continuous CSF drainage via ommaya reservoir is the answer? Neurology India 68:458-461
[Crossref] [Google Scholar] [PubMed]
- Vlachogiannis P, Hillered L, Enblad P, Ronne-Engstrom E (2022) Temporal patterns of inflammation-related proteins measured in the cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage using multiplex proximity extension assay technology. PLoS One 17:e0263460
[Crossref] [Google Scholar] [PubMed]
- Kwon JH, Sung SK, Song YJ, Choi HJ, Huh JT, et al. (2008) Predisposing factors related to shunt-dependent chronic hydrocephalus after aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc 43:177-181
- Black PM (1986) Hydrocephalus and vasospasm after subarachnoid hemorrhage from ruptured intracranial aneurysms. Neurosurgery 18:12-16
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences